5
Our scientists have utilizedour chemistry advancements to expand the therapeutic and commercial opportunities of our
pipeline. These advancements, alongwith themanufacturing and analytical processes that are the same for all of our drugs, shorten
our timeline from initial concept to the first human dosewhen compared to smallmolecule drugs.
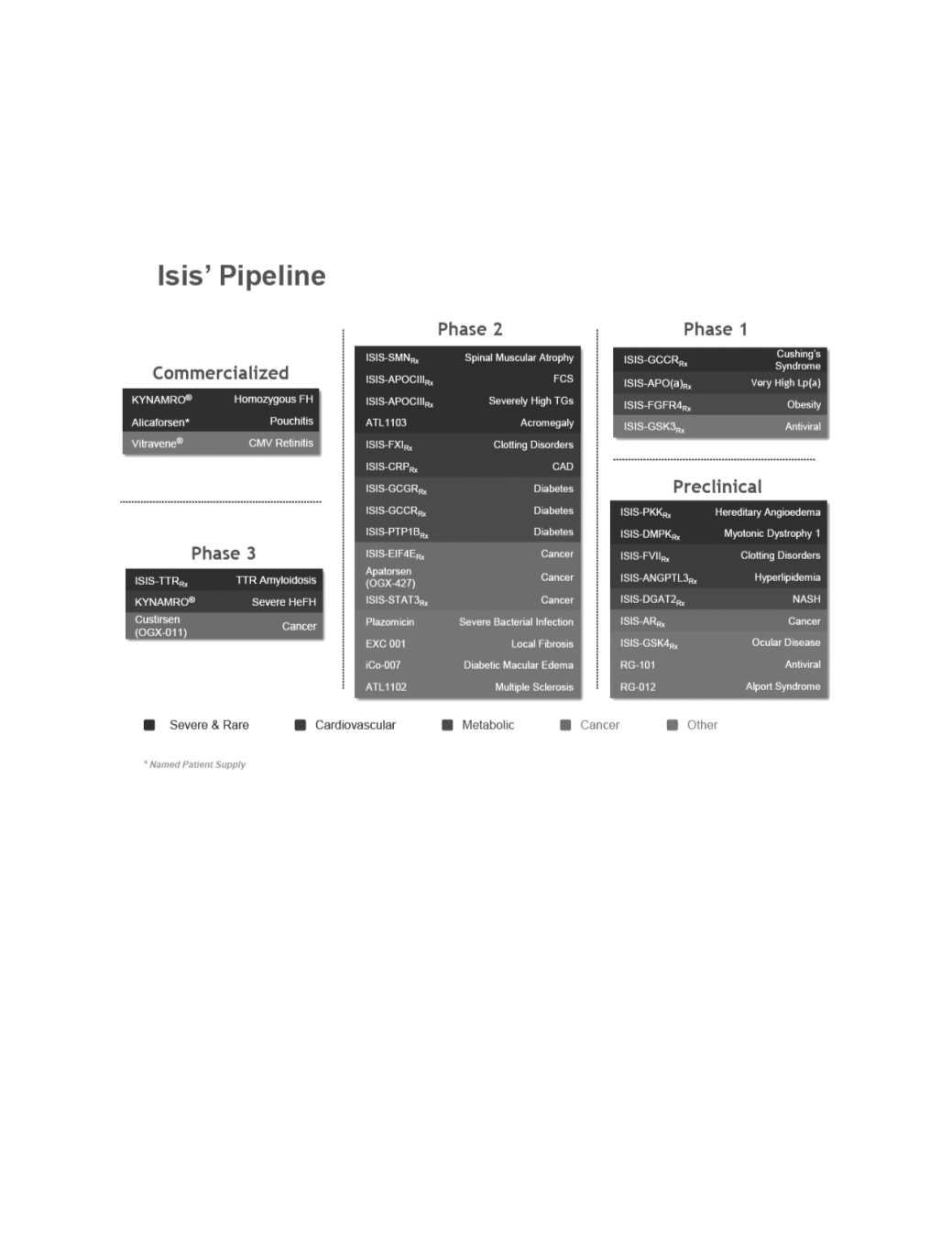
The following table lists our commercialized products and eachof our andour partners’ drugdevelopment projects, their
targets, disease indications and the development status of each. Typically, we identifyour drugs by the target, such as ISIS-APOCIII
Rx
or ISIS-GCGR
Rx
, and for some of our partnereddrugs, we refer to a drugby the partner’s own compoundnumber, such asATL1103
or iCo-007. As the drugs inour pipeline advance in clinical development, wewill adopt nonproprietarynames given to eachdrug
from theUnitedStatesAdoptedNamesCouncil. For example,mipomersen is a nonproprietaryname thatwe obtained for ISIS
301012 in2007. Oncewe or our partners establish a brandname, likeKYNAMRO formipomersen, wewill adopt the brandname.
KYNAMRO (mipomersen sodium) injection
Our flagshipproduct, KYNAMRO, is on themarket in theUnitedStates for patientswithHoFH. These are patientswho are
at high cardiovascular risk andwho are not able to reduce their LDL-C sufficientlywith currently available lipid-lowering therapies.
KYNAMROwas approvedby the FDA in January2013 as an adjunct to lipid-lowering therapy anddiet to reduceLDL-C,
apolipoprotein-B, or apo-B, total cholesterol andnon-high-density lipoprotein-cholesterol, or non-HDL-C, inpatientswithHoFH.
KYNAMRO is available in theUnitedStates under aREMSwith aBoxedWarning citing the riskof hepatic toxicity.
Genzyme is executing a comprehensive plan to address a global commercialmarket that consists of patientswho are in
desperate needof new treatment options forHoFH. AlreadyGenzyme has obtainedmarketing approval forKYNAMRO in theUnited
States, SouthKorea, Argentina andMexico for use inpatientswithHoFH and is continuing topursue approval inmultiple additional
markets. We believe thatGenzyme has the commercial infrastructure and ability to successfully commercializeKYNAMRO
worldwidemaking the drug available for patients inneed in approvedmarkets. Inorder to reach patientswithHoFH in theUnited
StatesGenzyme is concentratingmarketing and sales efforts on lipid specialists, cardiologists, andphysicianswho treat these types of
patients. In theUnitedStates, Genzyme has established theKYNAMROCornerstone, a programoffering services related toHoFH
andKYNAMRO, includingdedicated casemanagement, reimbursement support, financial assistance for thosewhoqualify, in-person
injection training, anddisease andproduct education for healthcare providers, patients, families, and caregivers. Genzyme also
continues to raise awareness ofHoFH. These activities include supporting continuedmedical educational programs to inform
physicians about HoFH andpartneringwithkey advocacygroups, such as Familial Hypercholesterolemia Foundation, theNational
LipidAssociation, AmericanCollege of Cardiology, International SymposiumonAtherosclerosis and theAmericanHeart
Association.


