metabolic abnormalities, such as insulin resistance and/or metabolic syndrome. In addition, people with elevated
triglycerides are at increased risk for type 2 diabetes, and people with severely elevated triglycerides are at high
risk for acute pancreatitis and other serious conditions. Results from our studies support our continued
advancement of ISIS-APOCIII
Rx
.
ISIS-APOCIII
Rx
is the most advanced drug in our lipid franchise. We plan to transition the development of
ISIS-APOCIII
Rx
toAkcea, our wholly owned subsidiary, which is also responsible for conducting commercial
activities. ISIS-APOCIII
Rx
is in development to treat patients with partial lipodystrophy and patients with FCS.
Both partial lipodystrophy and FCS are rare orphan diseases, and each one affects approximately one to two out
of a million people. Patients with partial lipodystrophy have diabetes and other metabolic abnormalities,
including elevated triglycerides, which increases their risk of pancreatitis. We believe that the robust triglyceride
reduction and the improvements in glucose control we observed in our Phase 2 program support our evaluation
of ISIS-APOCIII
Rx
in this patient population. FCS patients often have triglyceride levels higher than 2,000
mg/dL and experience a number of health problems such as recurrent acute pancreatitis that often requires
hospitalization, abdominal pain, and enlargement of the liver and spleen. We believe that the significant unmet
medical need for an effective triglyceride-lowering drug for patients with FCS and partial lipodystrophy and the
robust, consistent effects we observed with ISIS-APOCIII
Rx
should enable us to rapidly move this program
forward toward the market.
In preclinical studies, ISIS-APOCIII
Rx
diminished signs of metabolic syndrome and reduced atherosclerosis
in mice. In a Phase 1 study in healthy volunteers, ISIS-APOCIII
Rx
produced rapid, dose-dependent median
reductions in blood of up to 78 percent in apoC-III protein levels and up to 44 percent in triglyceride levels.
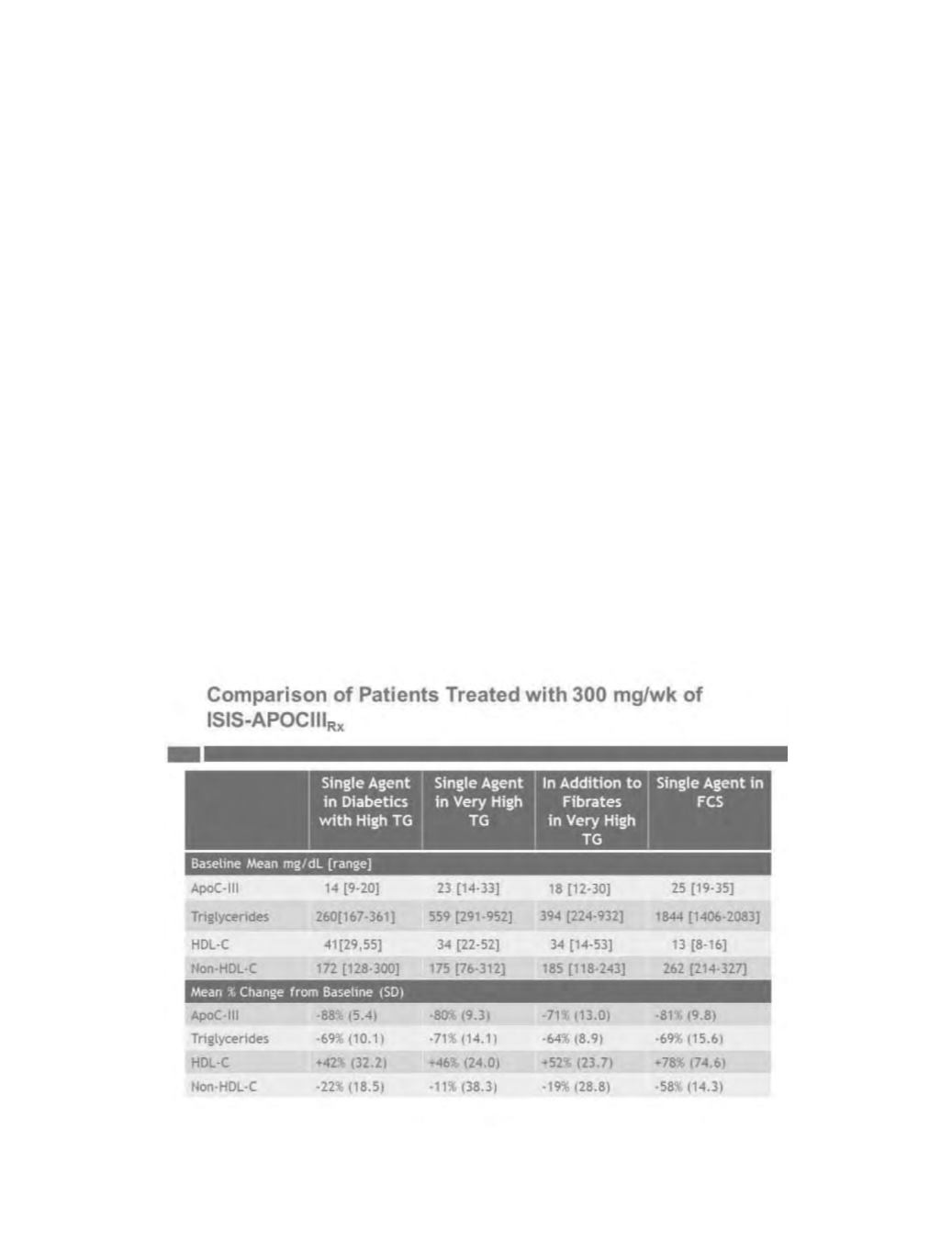
We completed a broad Phase 2 program evaluating ISIS-APOCIII
Rx
in patients with high, very high, and
severely high triglycerides, in patients with type 2 diabetes and in patients with FCS. We also evaluated
ISIS-APOCIII
Rx
both as a single agent and in combination with fibrates. Patients in our Phase 2 program entered
with baseline triglyceride levels ranging frommoderately high to severely high. In all patient groups treated with
ISIS-APOCIII
Rx
, irrespective of their incoming triglyceride levels, we observed consistent reductions in apoC-III,
triglycerides and apoC-III-associated very low-density lipoprotein, or VLDL, complexes, and increased HDL,
with a positive effect on non-HDL. Data from the 300 mg/week dose from each of these studies are summarized
in the table below.
15


