Migraine
Frovatriptan
2002 has witnessed a number of important milestones in the
Company's evolution as a product company, with the approval of
frovatriptan in 15 European Union countries, followed by its launch
as an acute treatment for migraine both in the United States
and in its first European country, Germany. In the United States,
frovatriptan had achieved a market share greater than 2% for the
triptan class of drugs nine months following launch, according to
prescription data generated by the market research organisation
IMS Health. In Germany, after 20 weeks on the market,
frovatriptan's market share of units sold had increased to
5.2% of the triptan class, also based on IMS Health data.
In the United States the product is being co-promoted by Elan
and UCB under the trademark Frova™, with sales forces detailing
to neurologists and primary care physicians.
In Germany, frovatriptan is being marketed by Berlin Chemie, the
German affiliate of our European licensee Menarini. Menarini has
also recently launched the drug in the United Kingdom and Ireland,
and is preparing for further European launches later in 2003.
In the third quarter of 2002, we announced positive headline
results from a multi-centre US clinical trial exploring frovatriptan's
potential use in the prophylaxis (prevention) of menstrually
associated migraine. This is a common form of migraine suffered
by women, which occurs at or around the time of menstruation.
It is estimated that over 50% of female migraineurs report
menstruation as one of the triggers for their migraine attacks.
Reports indicate that menstrually associated migraines tend
to last longer than other migraines and are more debilitating,
generally more resistant to treatment, and more likely to recur
after an initial response to treatment.
Our study, a double-blind, placebo-controlled, crossover design,
was conducted at 36 clinics in the United States and involved over
500 menstrually associated migraine (MAM) sufferers. Patients
were evaluated over three menstrual cycles over the course of
which each patient received all three dose regimens - placebo,
2.5mg frovatriptan once daily and 2.5mg frovatriptan twice
daily. During each cycle they took the treatment for a total of
six days commencing two days before the predicted onset
of their headache.
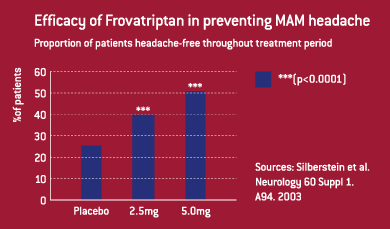
The headline results showed that both dose levels of frovatriptan
were highly effective in reducing the incidence, severity and
duration of menstrually associated migraines compared to placebo.
By the intention to treat analysis half the patients were completely
headache-free during the six-day period when they took 2.5mg
frovatriptan twice daily and around 40% when they took 2.5mg
frovatriptan once daily, compared to only 26% when they took
a placebo. The improvement in headache-free rates was highly
statistically significant for both dose regimens compared
to placebo (p<0.0001).
The full results of the study were presented at a meeting of the
American Academy of Neurology in the United States in April 2003,
and will subsequently be published in key scientific and clinical
journals. Additional studies will be required to enable Elan and
Menarini to apply for US and European regulatory approval for the
use of frovatriptan in the prophylaxis of menstrually associated
migraine. We believe that the requirements to submit applications
to extend European product licences will be broadly similar to
those in the United States.
No drugs of the triptan class are currently approved for
prophylactic use in menstrually associated migraine. If the positive
outcome of the initial study is reproduced in further studies and
an extension to the product licence successfully obtained, we
believe that it would give frovatriptan a competitive advantage
that could enable our marketing partners to significantly increase
frovatriptan's market share.
Sexual dysfunction
VML 670 - sexual dysfunction
There is increasing interest in new treatments for sexual
dysfunction. In our programme with VML 670 we are currently
targeting a specific male and female patient population whose
sexual dysfunction has arisen as a direct result of drugs that
they take to treat depression.
The most widely prescribed class of anti-depressant drugs
are known as SSRIs (selective serotonin re-uptake inhibitors).
They work by increasing circulating levels of serotonin (5-HT),
a chemical produced in the brain that affects mood. According
to published studies, a significant proportion of patients taking
SSRIs, between 30% and 40%, experience some form of sexual
dysfunction as a side effect of the treatment. This may manifest
itself in a number of ways, including loss of sexual desire, erectile
dysfunction, or an inability to achieve orgasm.
Studies have shown that SSRIs reduce the activity of one particular
type of receptor for serotonin, the so-called 5-HT1A receptor, which
is known to be involved in the mediation of sexual behaviour.
The hypothesis we are exploring is that VML 670, which has
potent and selective activity at 5-HT1A receptors, can re-activate
these receptors and so restore normal sexual function.
Our programme has made good progress throughout the year.
In the first half we successfully completed a Phase I programme
that included single and multiple-dose studies in healthy
volunteers as well as a study to confirm that there are no adverse
consequences from the co-administration of VML 670 and a
commonly prescribed SSRI anti-depressant. We subsequently
started a multi-centre, double-blind, placebo-controlled Phase IIa
trial with VML 670 in approximately 240 male and female patients
taking either of the two most commonly prescribed SSRIs.
Recruitment for the study, which we are running at 40 centres
in the UK, is now complete. We remain on track to review the
preliminary results with our partner, Lilly Corporation, in the
second half of 2003.
Parkinson's disease
Adenosine A2A antagonist programme
In our adenosine programme we are targeting two different clinical
indications, Parkinson's disease and depression.
Parkinson's disease
Parkinson's disease is a debilitating and progressive movement
disorder that affects approximately 1% of the population over the
age of 65 years. Common symptoms include stiffening of the
muscles and difficulty in initiating movement. Sufferers may also
develop a tremor or shaking. The primary cause of the problems
of co-ordination and movement in Parkinsonian patients is nerve
degeneration and cell death in the brain, leading to the loss of
dopamine, a chemical produced in the brain that is involved in
the control of voluntary movement.
Most conventional therapies for Parkinson's disease are based
on dopamine replacement. Although generally effective in the
short term, these treatments can have severe, or even disabling,
side effects and their effectiveness tends to decrease over time.
In addition, current therapies that target dopamine do not slow
down or stop progression of Parkinson's disease.
Adenosine is another brain chemical that also plays an important
role in motor co-ordination and movement control. A subtype of
adenosine receptor, the adenosine A2A receptor, is found in high
density in the part of the brain responsible for motor function,
where it appears to direct the activity of other neurotransmitter
mechanisms, including dopamine mechanisms, that are
dysfunctional in Parkinson's disease. We believe that by using
selective adenosine A2A receptor antagonists to restore the
imbalance of the other neurotransmitters caused by the loss
of dopamine, we may be able to provide a novel approach to
treat the symptoms of Parkinson's disease and to slow or stop
its progression.
During 2002 we selected our first lead development candidate
for Parkinson's disease, VR 2006. We are currently completing
a series of pre-clinical studies that are required by the regulatory
authorities to establish the safety profile of the drug before it can
be given to humans. Our intention is to take the compound into
clinical trials subject to additional funding becoming available
through a strategic collaboration or the implementation of another
strategic option.
Depression and anxiety
Depression and anxiety
Depression is the leading cause of disability worldwide. In the
United States, the world's largest market for pharmaceutical
products, it is estimated that depressive disorders affect
approximately 17 million people, and nearly twice as many
women (12%) as men (7%) are affected by a depressive
disorder each year.
In May 2002 we were very pleased to announce a new strategic
collaboration with Roche to research and develop A2A antagonists
as anti-depressants. This followed pre-clinical research by Vernalis,
Roche and a number of other companies suggesting that adenosine
receptors, and specifically the adenosine A2A receptor subtype, may
be a novel target for a completely new class of anti-depressant
drugs. In our own programme we have already synthesised
selective adenosine A2A receptor antagonists that show activity
in pre-clinical models of depression.
Under the terms of the agreement, Roche has an option to license
worldwide rights to develop, manufacture and market product
candidates coming out of our research programme for the
treatment of depression and anxiety, which is exercisable up to
the end of Phase I studies. On each anniversary of the option grant, Roche has to pay the Company an option renewal fee in order to
extend its option for a further year, and we also have the potential
to receive milestone payments for compounds that move into
development. If Roche exercises its option, the two companies
would pool their respective intellectual property and would
establish a joint research programme under which Roche would
fund our research costs. Roche would also then undertake and
fund all development work. We would potentially receive a series
of development and post-approval milestone payments totalling
up to $80 million on product candidates from the joint programme,
whether arising from Vernalis or Roche intellectual property,
as well as substantial royalties on future product sales.
In the prospectus for the Placing and Open Offer of 1 May 2003,
we stated that work on this programme would continue whilst we
pursue strategic options for the Company, but that the future of the
programme would depend on additional funding becoming available
either through a strategic collaboration or the implementation of
another strategic option.
Obesity
5-HT2C agonist programme - obesity
Over the last ten years there has been a dramatic rise in the
prevalence of obesity, particularly in first-world countries.
Obesity in the United States has reached epidemic proportions,
with over one-third of the total population now considered by the
National Institutes of Health to be clinically obese. This figure is
projected to exceed 50% within the next 25 years. In European
countries the corresponding figure is lower, approximately 20%,
but the rate of increase in prevalence is showing a similar trend
to that of the United States.
The important role of the 5-HT2C receptor in controlling eating and
satiety was a ground-breaking discovery by our own scientists in
1997. In obesity, this control mechanism may not work effectively
and may lead to excess food consumption, which the body stores
as fat. Compounds that activate this receptor can help to promote
the feeling of satiety and control the urge to eat to excess. Our
programme is focused on developing novel and highly selective
5-HT2C receptor agonists as drugs to improve weight loss.
Our 5-HT2C programme has been the subject of a joint research
and development collaboration with Roche since December 1999.
Roche's continued commitment to the programme was clearly evidenced by a new agreement that we signed with Roche
in February 2002. The new agreement extends the original
collaboration and includes significantly improved commercial
terms for Vernalis, with further research funding, potential
milestone payments from Roche now doubled to around
$60 million, and royalties rising to as high as 15% on
successfully marketed compounds. Roche will continue
to undertake and fund all development work.
The first clinical development candidate from this programme
entered Phase I clinical trials in May 2002, triggering a milestone
payment to Vernalis. We and Roche subsequently took the decision,
in July, to withdraw this compound from the Phase I trials, when
the early pharmacokinetic data in man revealed unexpectedly
high levels of a metabolite of the drug in the bloodstream.
Although there were no resultant side effects, the data did
not meet our stringent criteria for progression into Phase II.
While this was undoubtedly a setback, it is not unusual for drugs
to be discontinued in Phase I. The intention is to take at least one
compound from the programme into Phase I trials as soon as
possible. We currently anticipate a new development candidate
being selected in the second half of 2003.
|

|
|
