







|
 |



We believe in a balanced approach to developing new and improved cancer fighting drugs. First, we must build on today’s treatment options—cytotoxic agents that have served as the cornerstone of cancer care for decades. We are developing next-generation cytotoxics that widen the therapeutic window by increasing the anti-cancer benefit of these aggressive drugs while managing the harmful side effects. Second, we must continue to explore our growing understanding of the genetic basis for cancer with the development of mechanism-based therapeutic agents, designed to act on gene targets that are involved in the malignant progression of cancer. We believe that as we are making the transition to a new paradigm for the treatment of cancer, many of these novel targeted therapeutics will be effectively used in combination with conventional cytotoxic agents in order to maximize the therapeutic benefit and provide an array of effective treatment options for patients.
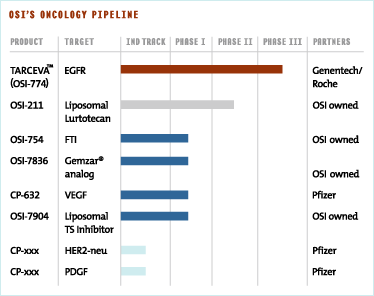
OSI has for many years conducted drug discovery efforts in targeted therapies. The first advanced drug candidate to emerge from these efforts is Tarceva™ (OSI-774), a small molecule inhibitor of the epidermal growth factor receptor (EGFR). Tarceva™ (OSI-774) is currently in Phase III clinical trials for non-small cell lung cancer and pancreatic cancer. We believe the acquisition of Gilead’s oncology assets will complement our drug discovery engine and accelerate our development and commercialization capabilities with the addition of a world-class drug development and oncology group. With this acquisition we have also augmented our pipeline of gene-targeted small molecule therapeutics with several promising next-generation cytotoxic agents currently in clinical development.
Today, OSI has a full array of cancer drug discovery and development capabilities that effectively differentiates our Company and will allow us to excel in our goal of becoming a global leader in oncology.


The discovery and development of new drug targets is crucial for the development of improved therapies for cancer. OSI pioneered the development of anti-cancer programs that target the multiple underlying mechanisms of cancer.
The most advanced of these programs targets the EGFR tyrosine kinase, a cell surface receptor with an important role in the control of cell growth and differentiation. Extensive studies show that in many human cancers, EGFR is over-expressed or mutated, leading to aberrant signaling and the development of tumors.

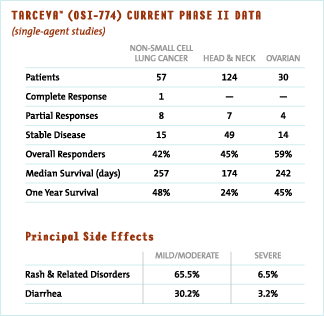

Tarceva™ (OSI-774), our small molecule anti-cancer agent, is a selective and orally active inhibitor of EGFR and is currently being tested in clinical trials. We have now completed Phase II trials for Tarceva™ (OSI-774) in non-small cell lung cancer, head and neck cancer and ovarian cancer. Patients in these trials had advanced or metastatic cancer and had generally failed standard treatment regimens. Objective evidence of anti-tumor activity manifested itself as partial responses and disease stabilization. The principal side effect seen for Tarceva™ (OSI-774) is an acneiform rash that is observed in approximately 72% of patients.
 Tracy Goad Walter—mother, wife, attorney, Tarceva™ (OSI-774) trial patient Tracy Goad Walter—mother, wife, attorney, Tarceva™ (OSI-774) trial patient
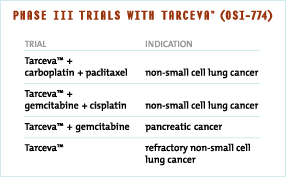
Since she was diagnosed with lung cancer in the Spring of 1998, Tracy had undergone a number of different cancer therapies. In July 2000, Tracy was enrolled in a single agent Phase I Tarceva™ (OSI-774) trial. Today, Tracy continues with Tarceva™ (OSI-774) as her daily treatment. Tarceva™ (OSI-774) is currently in Phase III trials in non-small cell lung and pancreatic cancers.
|
In January 2001, we entered into an alliance with Genentech and Roche for the global co-development and commercialization of Tarceva™ (OSI-774). This global program is designed as a broad-based approach in implementing several Phase III trials that we expect will result in an effective registration with the Food and Drug Administration and other international regulatory agencies. These trials include combination studies with existing chemotherapy regimens for front-line use in pancreatic cancer and non-small cell lung cancer as well as a single agent trial for refractory non-small cell lung cancer. We have initiated several Phase Ib combination trials to evaluate the effects of Tarceva™ (OSI-774) used in conjunction with several different chemotherapy drugs. We have also begun an exploratory Phase II trial in advanced breast cancer using Tarceva™ (OSI-774) as a single agent.
OSI is also collaborating with the U.S. National Cancer Institute’s Cancer Therapy Evaluation Program to conduct clinical trials with Tarceva™ (OSI-774) in multiple tumor types, including epithelial malignancies of the gastrointestinal and genitourinary tracts, gynecological malignancies and brain tumors.
Tarceva™ (OSI-774) and EGFR
Elevated numbers of EGF receptors have been detected on a variety of human tumors including breast, lung, head and neck, and colorectal cancers. The over-expression or mutation of these receptors leads to the abnormal stimulation of growth factor pathways resulting in unregulated cell signaling. Tarceva™ (OSI-774) is designed to specifically inhibit a signal transduction pathway by blocking the activity of the EGFR tyrosine kinase and targeting the underlying molecular changes that define the conversion of normal cells into a cancerous state. Tarceva™ (OSI-774) is designed to accomplish this by inhibiting the EGFR tyrosine kinase so that the aberrant signaling pathway is blocked and normal growth is restored.
|
Back to Top
We now know that cancers are caused by abnormalities in the expression of certain genes, especially oncogenes (growth regulating genes that are either over-expressed or mutated in cancer cells). The language of cancer is to be discovered in aberrations to the cell signaling pathways that control cell proliferation, apoptosis (programmed cell death), angiogenesis (the process of blood vessel growth), invasion and metastasis. At OSI, our discovery efforts in targeted therapies are directed toward the development of drugs that target the genetic abnormalities present in human cancers and their consequences. Specifically, our drug discovery programs in targeted therapy include programs in signal transduction, angiogenesis and apoptosis.
Ras genes are among the most frequently activated oncogenes in cancer. Different forms of the ras oncogene are referred to as H-ras, K-ras and N-ras. Ras is a protein that normally transmits signals (including growth signals) from the cell membrane to the cell nucleus. In order to mediate this signal, ras proteins have to be modified by an enzyme called farnesyl transferase.
In many forms of cancer, ras is mutated resulting in the constant transmission of signals irrespective of the presence of external stimuli. In fact, mutated forms of ras can be found in 30% to 40% of human cancers.
OSI-754 (formerly CP-609,754), an orally active inhibitor of farnesyl transferase, is designed to function as an anti-cancer agent by interfering with the ability of the ras protein to mediate signaling. We recently received full commercial rights to OSI-754, which was discovered in collaboration with Pfizer as part of our long standing alliance in cancer drug discovery. Pre-clinical studies show that inhibitors of farnesyl transferase are more effective at blocking the membrane recruitment of the H- and N- forms of the ras gene rather than the more common K-ras gene. We will therefore enter OSI-754 into a Phase II clinical trial for bladder cancer where mutant and over-expressed forms of the H-ras oncogene are known to be present.
OSI-754 and Bladder Cancer
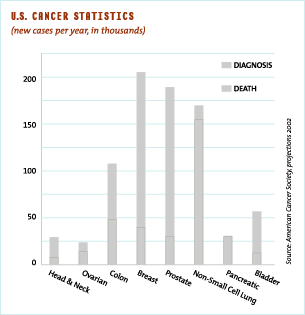
Bladder cancer is among the most common cancers in the United States. The American Cancer Society estimates that in 2002 there will be approximately 55,000 new cases of bladder cancer and about 13,000 deaths from this disease. With many bladder cancers exhibiting mutated forms of the H-ras oncogene, we recognize the opportunity to develop OSI-754 to address a specific and largely unmet medical need.
|
Angiogenesis, the process of blood vessel growth, has been shown to play an important role in the development and spread of cancer. Solid tumors induce angiogenesis because a blood vessel supply is necessary to provide nutrients to sustain tumor growth. In many cancers, the induction of angiogenesis is mediated by the production of a growth factor called vascular endothelial growth factor (VEGF). This growth factor binds to the VEGF receptor (VEGFR), a key tyrosine kinase involved in regulating blood vessel growth.A third drug candidate to enter clinical trials as a result of our successful cancer drug discovery alliance with Pfizer works by inhibiting the VEGFR. The compound is an orally active, potent and selective inhibitor of VEGFR and is currently in Phase I clinical trials, conducted by Pfizer.
We have additional drug discovery programs focused specifically on oncogenes and apoptosis, or programmed cell death. Normal cells undergo tightly controlled, or programmed death, which is often pathologically prevented in cancer cells. We are currently studying the mechanisms that underlie the ability of certain cells to avoid apoptosis and contribute to tumor growth by promoting cell survival.
Back to Top
Cancer therapy is undergoing a transition from the pre-genomic era of traditional cytotoxic drugs to a new era focusing on gene targeted therapies and combination approaches.
As these new targeted drugs emerge in clinical testing, they are frequently used in combination with cytotoxic chemotherapy drugs in an attempt to maximize the anti-cancer benefit. There is now a large body of experimental evidence showing that this type of combination therapy may be a promising therapeutic approach. We believe that a successful oncology franchise should develop both next-generation or improved cytotoxic drugs in addition to the new targeted therapies in order to provide an array of treatment options for the cancer patient.
Thus, while our drug discovery research efforts have focused primarily on targeted therapies, our acquisition of oncology assets from Gilead has further complemented our portfolio with several promising, next-generation cytotoxic agents. These include two compounds incorporating novel liposomal formulations—OSI-211 (formerly NX211), a topoisomerase I inhibitor, and OSI-7904 (formerly GS7904L), a thymidylate synthase inhibitor. Non-liposomal formulations of these products are currently marketed for cancer indications. Additionally, OSI-7836 (formerly GS7836) is being developed as a next-generation GemzarÆ (gemcitabine) for multiple solid tumors.
OSI-211 is a proprietary liposomal formulation of the anti-cancer agent, lurtotecan, a topoisomerase I inhibitor. This novel formulation is designed to enhance efficacy and improve the drug’s therapeutic effect. Topoisomerase I is an enzyme critical to cellular replication. Specifically, topoisomerase I enables double-stranded DNA to unwind prior to replication. Failure of DNA to unwind appropriately during the cell cycle sends a cell into apoptosis, preventing further cell replication. HycamptinÆ and CamptosarÆ are examples of currently marketed topoisomerase I inhibitors. Phase I studies of OSI-211 revealed some promising indications of anti-tumor activity in ovarian and other cancers. Phase II clinical trials are in progress for patients with ovarian and small cell lung cancers.
OSI-7904 is a member of the class of drugs known as thymidylate synthase inhibitors, a well established group of agents with a validated mechanism of action, often used in metastatic colorectal and breast cancers. 5-Fluorouracil and XelodaÆ are examples of marketed drugs in this class. This liposomal formulation is designed to improve the therapeutic window and enhance activity by maintaining active levels of drug in the tumor for extended periods of time. This product is currently in Phase I trials.
OSI-7836 is a member of the nucleoside class of cytotoxic drugs of which the most prominent is GemzarÆ, which has been approved for use in front-line combinations for non-small cell lung cancer and for use in pancreatic cancer. OSI’s next-generation product has been shown to be more active than GemzarÆ in pre-clinical xenograft models of anti-tumor activity and is currently in Phase I trials.
Core Drug Discovery Research
We also intend to continue to discover and develop small molecule drugs that address other major markets outside of cancer. In these efforts we expect to focus primarily in the areas of diabetes and obesity, taking advantage of our extensive research expertise in this field. Our historical partnerships have resulted in a pipeline of drugs being developed by Tanabe (diabetes), Aventis (cholesterol lowering and asthma), Pfizer (cosmeceuticals) and Solvay (congestive heart failure) that could yield royalty income to OSI if they are successful in reaching and gaining marketing approval.
|
Back to Top
|
|
 |

|
|








 Tracy Goad Walter—mother, wife, attorney, Tarceva™ (OSI-774) trial patient
Tracy Goad Walter—mother, wife, attorney, Tarceva™ (OSI-774) trial patient