BIOPURE CORPORATION has pioneered the
development and manufacture of a new class of pharmaceuticals,
called oxygen therapeutics, which are intravenously
administered to deliver oxygen to the body’s
tissues. The company’s products represent a new
Oxygen Bridge™ treatment approach for managing
patients’ oxygen requirements in a broad range of
potential medical applications, including their use as an
alternative to red blood cell transfusion in treating acute
anemia, as an adjunct to cancer therapy and as a means
of preventing tissue damage and organ dysfunction in
ischemic conditions such as heart attack and stroke.
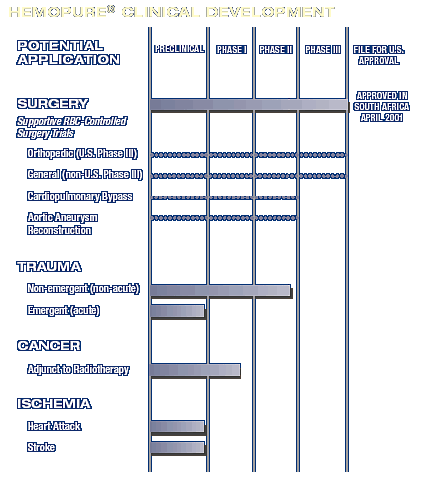
RECENT MILESTONES
- World’s first regulatory approval of Hemopure®
granted in South Africa
- Primary endpoints met in U.S. Phase III clinical trial
of Hemopure
- Sales of Biopure’s veterinary product Oxyglobin®
surpassed 100,000 units since its introduction in 1998
- $75 million financing agreement entered into with
Société Générale
- $120 million financing letter of intent signed for new,
large scale manufacturing facility in Sumter, S.C.
- Began expanding production capacity of existing
manufacturing facility in Cambridge, Mass.
- Two additional U.S. patents issued