 The
strong operating performance of American Home Products in 2000 reflects
AHP's successful evolution into a world leader in research-based pharmaceutical
products. In June, we completed the divestiture of our agricultural
products business. AHP now is completely focused on pharmaceuticals,
consumer health care products and animal health products. Each of
these businesses achieved excellent results during the year, enabling
our Company to increase net revenue, on a pro forma basis, by 13 percent.
With leading products in important market segments and strengths in
product discovery, development, manufacturing and marketing, we anticipate
solid growth for AHP well into the future. The
strong operating performance of American Home Products in 2000 reflects
AHP's successful evolution into a world leader in research-based pharmaceutical
products. In June, we completed the divestiture of our agricultural
products business. AHP now is completely focused on pharmaceuticals,
consumer health care products and animal health products. Each of
these businesses achieved excellent results during the year, enabling
our Company to increase net revenue, on a pro forma basis, by 13 percent.
With leading products in important market segments and strengths in
product discovery, development, manufacturing and marketing, we anticipate
solid growth for AHP well into the future.
AHP's revenue growth in 2000 underscores the strength of our global
pharmaceutical business. Today, more than 81 percent of net revenues
are from pharmaceuticals - up from 51 percent just 10 years ago.
AHP's stock price in 2000 benefited from the Company's operating performance,
out-pacing major competitors in the pharmaceutical industry, as well
as the Standard & Poor's 500, the Dow Jones Industrial Average and
the NASDAQ, by a wide margin.
AHP's performance in new product launches was among the most impressive
in the industry. Wyeth-Ayerst, our ethical pharma-ceutical division,
received regulatory approval for seven major pharmaceutical and vaccine
products from June 1999 to May 2000, the best new product approval
record in the industry during that time period. These innovative
new products reflect success from all three of our discovery and development
platforms: small molecules, proteins and vaccines.
We took aggressive action during 2000 to move toward resolution of
the diet drug litigation involving AHP. In August, AHP received trial
court approval of the negotiated nationwide, class action settlement
of the litigation, which covers the vast majority of the individuals
who took AHP's diet drugs. Among patients who opted out of the settlement,
approximately 80 percent of claims now have been settled or are subject
to settlement agreements. In the fourth quarter of 2000, the Company
recorded an additional charge of $7.5 billion related to the litigation,
bringing the total charges for the diet drug litigation to $12.25
billion. We are confident that no further charges will be required.
Although this total is more than originally expected, we believe it
is in the best interest of AHP stockholders to resolve this litigation
quickly. By working to put this matter behind us, our strong pipeline
and accelerating operating momentum no longer will be overshadowed,
and we can devote full attention to growing our pharmaceutical business
and capitalizing on our many global opportunities.
.
Net Revenue and Results of Operations
AHP's worldwide net revenue for 2000 reached $13.3 billion, an increase
of 13 percent over 1999 pro forma net revenue. Excluding the negative
impact of foreign exchange, pro forma worldwide net revenue increased
16 percent for the year. This increase was due primarily to growth
in pro forma global pharmaceutical net revenue of 15 percent in 2000.
Excluding the diet drug charge and other unusual items detailed in
the financial section of this report and including the dilutive effect
of common stock equivalents, both income and diluted earnings per
share from continuing operations increased 18 percent - from $2.13
billion and $1.61, respectively, for 1999 to $2.51 billion and $1.90,
respectively, for 2000.
Also in 2000, we reached an agreement with Immunex Corporation-in
which AHP was the majority stockholder-to participate in a stock
offering that would allow Immunex, our partner in the promotion of
Enbrel, to raise funds for additional research and manufacturing capabilities
while enabling AHP to realize a portion of the gain on its highly
successful investment in the company. In November, we completed the
sale of more than 60 million shares of Immunex stock in an underwritten
public offering, reducing AHP's ownership of outstanding shares from
55 percent to approximately 41 percent at the end of 2000. The Company
recorded an after-tax gain of $1.4 billion related to the sale. AHP
continues to be a major participant in the future development and
growth of Immunex. Importantly, our co-promotion partnership with
Immunex for the break-through rheumatoid arthritis (RA) therapy, Enbrel,
remains in full effect going forward.
Investing in the Future
AHP invested approximately $1.7 billion in research and development
in 2000. This investment is focused on key therapeutic franchises
that offer a strong foundation for growth and innovation.
We also are making major capital investments to provide AHP with
a competitive edge in biotechnology and protein manufacturing. For
example, we anticipate spending more than $2 billion over the next
five years to expand our manufacturing capacity for recombinant protein
and vaccine products at four locations - Andover, Massachusetts; St.
Louis, Missouri; Sanford, North Carolina; and West Greenwich, Rhode
Island-and to build a new state-of-the-art biopharmaceutical development
and manufacturing facility in Grange Castle, Clondalkin, Ireland.
In addition to our own research programs, we have established key
alliances with a number of other major pharmaceutical and biotechnology
firms. Two important alliances were announced in July 2000, when we
reached agreements with Celera Genomics Group and Incyte Genomics,
Inc. Through these relationships, AHP will gain access to databases
containing extensive information on human, animal and microbial genes,
including Celera's complete sequence of chemical "letters" of DNA
that make up the human genome.
As a result of these investments and alliances, AHP now has one of
the largest biotechnology research programs in the pharmaceutical
industry. Using genomics, recombinant DNA and other technologies,
AHP scientists are developing new strategies and innovative products
in the fight against some of the most devastating diseases, including
cancer, Alzheimer's, diabetes and hemophilia.
In October 2000, Wyeth-Ayerst entered into a consent decree with the
U.S. Food and Drug Administration (FDA) focusing on the Company's
compliance with current Good Manufacturing Practices at our facilities
in Marietta, Pennsylvania, and Pearl River, New York. We are confident
in the safety and efficacy of our products and feel certain that the
investments we are making to improve our manufacturing processes and
facilities will help us to consistently maintain the highest quality
standards.
In 2001, we will begin the relocation of employees into the new Wyeth-Ayerst
global headquarters in Collegeville, Pennsylvania, which we purchased
in 2000. The Collegeville facility will provide a fully integrated
campus environment that will enhance efficiency and communication.
Human Ethical Pharmaceuticals
The strong growth of AHP's human pharmaceutical business in 2000
reflected the impact of new product launches as well as the continuing
strength of our cornerstone global products.
Sales of the Premarin family of hormone replacement therapy
products approached $1.9 billion for the year. Worldwide sales of
the Effexor family of antidepressants reached nearly $1.2 billion
in 2000 - a 48 percent increase over 1999. Enbrel achieved
$690 million in global sales. Wyeth-Ayerst continued to expand these
key product lines in 2000 with new claims, indications and dosages:
Effexor XR was approved in the United States for the long-term
treatment of generalized anxiety disorder; Enbrel received
FDA approval for inhibiting the progression of structural damage in
the joints of early stage RA patients; and regulatory submissions
were filed for new, lower dose formulations of Premarin and
Premarin/MPA products.
AHP's new products also produced significant results in 2000. The
launch of Meningitec, a meningococcal Group C conjugate vaccine,
was advanced to reach the market in the United Kingdom in October
1999, enabling the U.K. Department of Health to initiate a vaccination
program before the 1999-2000 winter season. Meningococcal disease
is one of the most common causes of death in children and young people
under the age of 20 in the United Kingdom. In January 2001, the U.K.
Department of Health reported a 90 percent reduction in the number
of meningococcal Group C cases in the age group at highest risk since
the inception of the vaccination program.
Prevnar, the first vaccine to help prevent invasive pneumococcal
disease in infants and young children, has been well-received in both
the private and public health sectors following its recommendation
for infant immunization. After FDA approval in February 2000, Wyeth-Ayerst
shipped more than 9 million doses of Prevnar for a total of
$461 million in sales in 2000. European Union approval of the vaccine
- marketed as Prevenar internationally - was received in February
2001.
In February 2000, the FDA approved Protonix for short-term
treatment in the healing and symptomatic relief of erosive esophagitis.
Following a May 2000 launch, Protonix had a successful first
year on the market with $145 million in sales.
In March 2000, ReFacto, for hemophilia A, was approved by the
FDA, and the product was launched in the United States in January
2001.
In addition, Wyeth-Ayerst's novel oncology therapy, Mylotarg,
was approved in the United States in May 2000 for the treatment of
relapsed acute myeloid leukemia in patients over age 60.
Altace, an ACE inhibitor co-promoted in the United States by
Wyeth-Ayerst and King Pharmaceuticals, Inc., received FDA approval
in 2000 for an important new indication-to reduce the risk of stroke,
heart attack and death from cardiovascular causes in patients over
age 55 at risk for cardiovascular disease.
Additionally in 2000, a regulatory submission was accepted for review
in the United States for FluMist, an intranasal flu vaccine.
Regulatory review of rhBMP-2, a unique recombinant protein that stimulates
bone growth to facilitate the healing of long-bone fractures that
require open surgical management, began early in 2001.
Consumer Health Care
Whitehall-Robins Healthcare continues to be a leader in the global
consumer health care market. Total sales in 2000 were nearly $2.5
billion, driven by increased sales in our three largest consumer health
care categories - analgesics, cough/cold/allergy products and vitamins/nutritional
supplements. Ten of the division's products rank number one or two
in their category in the United States, and two global consumer health
care brands - Advil and Centrum - are among the top
10 selling consumer health care brands in the world.
Animal Health Products
AHP's Fort Dodge is a global leader in the animal health industry.
Fort Dodge sales in 2000 reached nearly $800 million, an increase
of 20 percent over 1999. Fort Dodge has expanded recently through
innovative product development, supplemented by a series of strategic
acquisitions. Product introductions during the year included the launch
in Australia of ProHeart SR12, a groundbreaking, once-a-year
injectable for the prevention of heartworms in dogs, which is expected
to enter the U.S. market in 2001.
Inside AHP
On May 1, 2001, Robert Essner will become the Chief Executive Officer
of the Company and will continue as President of the Company, a position
he was elected to in July 2000. John R. Stafford will remain Chairman
of the Board, and Mr. Essner will continue to serve on the Board.
Mr. Essner has been instrumental for the past 11 years in leading
our pharmaceutical business. We believe that his election as the next
Chief Executive Officer provides for continuity of our strategic
direction as a first-tier pharmaceutical company devoted to the development,
manufacturing and marketing of a broad range of innovative products.
Several other significant appointments were made within the last 12
months. In July, Louis L. Hoynes, Jr., was elected Executive Vice
President and General Counsel of AHP. He previously was Senior Vice
President and General Counsel of AHP. Additionally at AHP, L. Patrick
Gage, Ph.D., was elected Senior Vice President-Science and Technology;
Bernard J. Poussot was elected Senior Vice President; Lawrence V.
Stein was elected Vice President and Deputy General Counsel; and Justin
R. Victoria was elected Vice President-Investor Relations.
Changes in AHP's Board of Directors during 2000 include the election
in July of Richard L. Carrión, who serves as Chairman, President and
Chief Executive Officer of Banco Popular de Puerto Rico -the leading
banking institution in Puerto Rico -and of Popular, Inc., Banco Popular's
holding company; and the election in October of Walter V. Shipley,
retired Chairman of the Board of The Chase Manhattan Corporation.
In 2000, three officers of AHP-Joseph J. Carr, Gerald A. Jibilian
and William J. Murray-retired after many years of dedicated service.
We thank these gentlemen for their many contributions to the Company.
It is with great sadness that we note the passing of Robert I. Levy,
M.D., Senior Vice President - Science and Technology for AHP, in October
2000. Dr. Levy was a giant in the field of medicine and in the pharmaceutical
industry, and his leadership was invaluable in guiding AHP through
its evolution into a leading research-based global pharmaceutical
company.
Moving Forward
AHP is moving forward with confidence in its 75th anniversary year
as a strong, independent company well-positioned for continued growth
in the global pharmaceutical marketplace. We recently have launched
a significant number of new pharmaceutical products, and we continue
to invest in our research and manufacturing capabilities to sustain
our growth in the future. More important, the drugs we produce are
saving lives and improving the quality of life for people around the
world.
In the pharmaceutical business, we recognize a special obligation
to our customers. From molecule to market, we must produce medicines
of the highest quality. This obligation is at the heart of AHP's
commitment to continuous improvement. To realize our vision for quality,
we are making substantial investments in plants, systems and people.
We thank our employees for their diligence and dedication, which were
the keys to our success in 2000. Their hard work, innovative ideas
and commitment to quality will maintain our momentum and help us
achieve even greater results in the years ahead.
We also thank AHP's Board of Directors for their guidance and support.
Our future is exciting. We look forward to applying our growing knowledge
of the human genome and the mechanisms of disease in the search for
new cures and innovative therapies for life-threatening diseases and
other challenging health problems on a global scale.
John R. Stafford
Chairman and Chief Executive Officer
Robert Essner
President and Chief Operating Officer
March 6, 2001
|
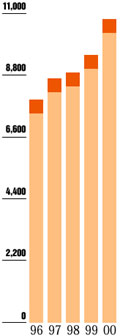
Pharmaceutical Net Revenue
$ millions |
|
|
"Today, more
than 81 percent of net revenues are from pharmaceuticals
- up from 51 percent just 10 years ago."
|
|
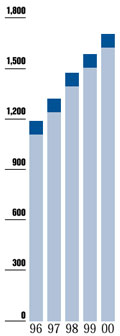
Pharmaceutical and Consumer Health Care R&D Expenditures
$ millions |
|
|
"AHP now has
one of the largest biotechnology research programs
in the pharmaceutical industry."
|
|
|
"AHP invested
approximately $1.7 billion in research and development
in 2000."
|
|
 YEARS OF INNOVATION
YEARS OF INNOVATION
|
American Home Products
Corporation was formed in 1926 through the merger
of a group of non-prescription medicine companies.
From its inception, the Corporation was successful
in building stockholder value. To illustrate-assuming
no dividend reinvestment -a $1,000 investment
in AHP stock when it went public in 1926 would
be worth nearly $2.5 million today. The same investment
in the S&P 500 -without dividend reinvestment-would
be worth approximately $0.1 million.
Over its 75-year history, AHP consistently has
invested in its health care businesses, leading
to significant accomplishments such as the mass
production of penicillin during World War II and
the development and man- ufacturing of vaccines
that virtually have eliminated smallpox and polio
globally.
In recent years, AHP has developed a leading expertise
in biotechnology and substantially increased its
pharmaceutical R&D spending, expanding its pipeline
of high-tech pharmaceutical products and delivering
many new, first-in-class therapies for serious,
unmet medical needs to the global market. |
|
|
|