
ANNUAL REPORT 2002
|
|
|
|
|
 |


|
BUSINESS DEVELOPMENT
SOUTH AFRICA
|
As the first country to grant regulatory approval of Hemopure for human use, South Africa has a head start on the rest of
the world in learning how oxygen therapeutics can revolutionize medicine. We at Biopure South Africa Ltd. look forward in
2003 to beginning product sales and integrating this new class of drug into the unusual environment that is South African
healthcare. While we are focused on the initial surgery market for Hemopure, we are also preparing to expand Biopure's
clinical development program in trauma into South Africa, which to our regret is often considered the trauma capital of
the world. Juxtaposed within this developing nation is

|

|
a country struggling to deliver basic primary healthcare to rural communities
while simultaneously providing a level of sophisticated medical service in urban centers that is unparalleled in most of the
world. Understanding the key drivers within this setting is critical to gaining acceptance and widespread use of Hemopure
as a revolutionary product and new treatment paradigm. While positioning the product as an alternative to blood makes sense,
simply placing it on the shelf and saying that the clinicians now have a "blood substitute" does not do justice to the
product's unique characteristics.

|

|
|

|

|
Therefore, we at Biopure South Africa are facing the challenge of introducing an innovative, but relatively expensive new
pharmaceutical product into the South African market by identifying its overall cost benefits and therapeutic value as an
oxygen therapeutic. To that end we are building a pharmacoeconomic model for payors, a medical readiness model for mining
groups and the military, and a clinical applications model for a broad range of medical specialties.

South African doctors relish the idea of having a treatment alternative for the management of surgical anemia, and they are
beginning to realize that Hemopure's ability to transport oxygen to the

|

|
microcirculation via the plasma suggests much broader
applications for the product. Improved wound healing, greater tumor penetration, reduced reconstructive flap problems, reduced
hospital stays, improved graft behavior and faster ventilator weaning are being thought about, talked about and observed!
One of our goals for 2003 is to have South African doctors begin sharing these experiences with the worldwide medical
community in appropriate medical forums.

Charl van Loggerenberg, M.D.
Managing Director
Biopure South Africa Ltd.

|

|

|
CLINICAL EXPERIENCE
SOUTH AFRICA
|
My clinical experience and impressions of Hemopure during the past year have been extremely positive. The ability to
safely enhance oxygen delivery to tissues at the microvascular level has suggested significant improvement in patient
outcomes and local tissue morbidity and has elevated the quality of surgical care we can offer patients undergoing
breast cancer surgery.

Dr. Gereth Edwards and I have reported on the first intraoperative use of Hemopure, outside of a clinical trial, in
25 patients to manage acute blood loss during major surgery aimed at curing malignancy. Our report focuses on the use of

|

|
undergoing mastectomy and immediate breast Hemopure in breast cancer patients
reconstruction, without blood usage, to reduce or eliminate the immune modulation effects associated with
conventional blood products.

The results—an extremely low wound complication rate and 100 percent flap healing—are impressive and suggest that
Hemopure could potentially eliminate wound complications and normal blood requirements, thereby contributing to
the establishment of a new standard of care in South African breast cancer patients.

|

|

|

|
|


Gereth Edwards, M.D.
Plastic and Reconstructive Surgeon
Milpark Hospital
|

|
"Without Hemopure, my patients would be exposed to high-risk blood transfusions and, in my opinion, longer recuperation periods and slower wound healing."
|

|

|
We concluded that these results were achieved by eliminating the immune modulation effects associated with blood transfusion
and by improving tissue oxygen delivery with Hemopure—an absolute delight for both the surgeon and the patient.

Carol Benn, M.D.
General Surgeon and Breast Cancer Specialist
Milpark Hospital, Johannesburg, South Africa

The South Africa package insert for Hemopure is available online at http://www.biopure.com/hemopurepi/rsa or per request by calling (617)234-6863.

|

|

|

HEMOPURE®
CLINICAL DEVELOPMENT
|

|

|

|

PRODUCT
CHARACTERISTICS
|
| CHARACTERISTICS |

|
BIOPURE'S
OXYGEN THERAPEUTICS |

|
RED BLOOD CELLS |

|
| STORAGE |

|
Room temperature (2º to 30º C) |

|
Refrigerated |

|

|

|
| SHELF LIFE |

|
36 months |

|
42 days |

|

|

|
| PREPARATION |

|
Ready to use |

|
Testing, typing and crossmatching |

|

|

|
| COMPATIBILITY |

|
Universal |

|
Type specific |

|

|

|
| EFFECTIVENESS |

|
Immediate oxygen delivery |

|
Dependant on length of storage |

|

|

|
| PURITY |

|
Processed to remove all potential contaminants |

|
Tested and screened for specific infectious agents |

|

|

|
| RAW MATERIAL |

|
Bovine hemoglobin - abundant, controlled source |

|
Human blood - limited availability, not controlled |

|

|

|
HEMOPURE®
CLINICAL DEVELOPMENT
|
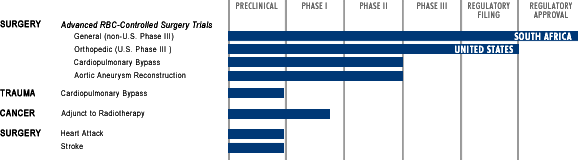
The surrounding tables describe our 22 clinical trials of Hemopure, the status of our target applications, and key product
characteristics as compared to allogeneic red blood cells. We began testing Hemopure in healthy volunteers 11 years ago
and have progressed through Phase I, II and III clinical trials focusing primarily on surgical patient populations, which
constitute 14 of the trials in the table below.

On October 1, 2002, the FDA accepted for review our biologic license application to market Hemopure in the United States
for the treatment of the signs and symptoms of acute anemia in adult patients undergoing elective orthopedic surgery,

|

|
and for the purpose of eliminating or reducing the need for allogeneic red blood
cells in these patients. Based on FDA performance goals and guidelines in the Prescription Drug User Fee Act (PDUFA),
we anticipate that the agency will complete its review and act on our BLA in mid 2003.

The clinical section of the BLA is based on four major, red blood cell controlled surgery trials—a Phase II study
in cardiac surgery, a Phase II study in vascular surgery, a non-U.S. Phase III study in non-cardiac general surgery,
and a U.S. Phase III orthopedic surgery study that, to our knowledge, is the only U.S. Phase III trial of a hemoglobin solution ever completed. The BLA is also based on

|

|
|

|
HEMOPURE®
CLINICAL TRIALS
|
| # STUDIES |
TYPE |
HEMOPURE SUBJECTS
(n) |
CONTROL SUBJECTS
(n) |
MAXIMUM TOTAL DOSE
(grams of hemoglobin) |

|
| 4 |
Healthy Volunteers |
64 |
29 |
45 - 140 |

|

|

|
| 4 |
Non-Surgery |
34 |
14 |
43 - 1080 |

|

|

|
| 3 |
Surgery with ANH* |
31 |
36 |
36 - 98 |

|

|

|
| 7 |
General Surgery |
146 |
95 |
27 - 300 |

|

|

|
| 4 |
Major Surgery Trials |
531 |
487 |
120 - 300 |

|

|

|
| 22 |
|
806** |
661 |
27 - 1080 |

|
* ANH = Acute Normovolemic Hemodilution
** Total does not include compassionate use patients treated under emergency INDs
|

|

|

|
HEMOPURE®
CLINICAL DEVELOPMENT
|
an integrated database for all of our trials, which includes more than 1400 total subjects of whom more than 800 were
administered Hemopure.

During the past 18 years more than 180 preclinical animal and laboratory studies have been conducted with Biopure's
oxygen therapeutics. These studies include the standard battery of toxicology tests required to support Biopure's
application. They also include pharmacology studies investigating the kinetics of oxygen onloading and offloading, tissue
oxygenation and the product's effects in various animal models of physiologic stress, including
hemorrhagic shock and trauma.

|

|
MILITARY TRIALS FOR TRAUMA SUPPORT In March 2002, Biopure Vice Chairman and Chief Technology Officer Carl
W. Rausch was invited to testify before a Congressional Armed Services Subcommittee at a hearing on innovative
technologies that support homeland defense and the war on terrorism. Because Hemopure can be stockpiled, positioned
abroad, and carried or stored in remote locations for immediate use when red blood cells are not readily available, it could potentially enhance
medical readiness and improve treatment in critical care conditions and in emergency situations on the battlefield.

|

|

|

|
|
In September 2002, the U.S. Army awarded Biopure a $908,900 grant, funded by the Defense Appropriations Act of
2002, for a randomized, standard therapy-controlled, Phase II clinical trial of Hemopure in consenting trauma
patients as a precursor to broader trauma trials. In November 2002, a committee of military and civilian trauma
experts reviewed data from the Phase III orthopedic surgery trial and the integrated safety database of all
Hemopure trials. During this meeting, the committee recommended broadening the proposed
Phase II trauma program to include more severely injured and unstable patients in emergency room and pre-hospital
settings. We

|

|
expect to receive up to $4 million in additional funding for this trauma program from monies in the
Defense Appropriations Act of 2003.

In addition, Biopure and the Naval Medical Research Center (NMRC) have tentatively agreed to the terms of a
Cooperative Research and Development Agreement (CRADA) for a multicenter Phase III trauma trial of Hemopure.
Biopure and NMRC are also cooperating on preclinical studies using Hemopure in the development of next-generation
hemoglobin-based oxygen carriers under the Navy's already funded Hematomimetics Program.

|

|

|
OXYGLOBIN®
|
Since Oxyglobin was approved by the FDA in 1998 and the European Commission in 1999, we have sold more than
115,000 units for the treatment of canine anemia regardless of the cause, which includes blood loss, disease
and ineffective red blood cell production. This veterinary experience provides a compelling proof of concept
for the valuable role oxygen therapeutics may soon play in human medicine.

While our manufacturing plant expansion and revalidation created a backorder situation for our veterinary
customers in 2002, in early 2003 we received FDA approval of our expanded facilities and promptly filled backorders in excess of

|

|
11,000 units. We are continuing to manufacture new product and are
committed to providing an uninterrupted supply of product now that shipments have resumed.

Our goals are to restore and expand Oxyglobin sales by launching new marketing and medical education
initiatives and by introducing a 60-milliliter bag that will give veterinarians greater flexibility in
matching the dose to the patient's oxygen requirements, particularly in smaller dogs. Internationally,
we will gradually expand Oxyglobin's availability in the European Union and target Japan as a future
veterinary market.

|

|

|

|
INJURED POLICE DOG
TREATED WITH OXYGLOBIN
|
On August, 22, 2002, Fresno Police Department K-9 Officer Saxon was shot at point blank range with a shotgun
blast from a felony suspect. The gunshot nearly proved fatal to K-9 Saxon as it punctured his lungs, broke two
legs, and ripped open his entire left side. Conversations between members of my staff and veterinarian Dr.
Roger Gfeller leave no doubt about the pivotal role that Oxyglobin played in saving K-9 Saxon's life.

The enormous boost to the morale of the K-9 Unit, the Fresno Police Department, and the community at large when
it was announced that K-9 Saxon would recover was evident in the events that

|

|
followed: school children writing get well cards; citizens sending in cards and donations on K-9 Saxon's behalf; other
law enforcement agencies' K-9's coming to the aid of our agency.

I would be honored if you would pass along my sincerest thanks to all of Biopure Corporation's employees who
have made Oxyglobin the life saving product that it is. Thank you again for your role in giving K-9 Saxon
back to a grateful community.

Jerry Dyer
Chief of Police, Fresno, California
November 26, 2002

|

|
|
 |
|