
|
 |

 The BD SoloShot IX The BD SoloShot IX
 and
BD SoloShot LX and
BD SoloShot LX
 autodisable
syringes autodisable
syringes
 expand
the expand
the
 BD SoloShot family BD SoloShot family
 to
five color-coded to
five color-coded
 syringes
for easy syringes
for easy
 identification. identification. |
 nnovation rarely comes to Afghanistan, Mali, Rwanda and
dozens of other developing countries. When it does–in the
form of deceptively simple, inexpensive injection devices–it's
easy to overlook. But what can't be overlooked is the impact
of auto-disable injection technology, not only in getting vaccines
to remote and rugged areas, but also in making progress
against unsafe immunization practices that risk transmitting
blood-borne pathogens. nnovation rarely comes to Afghanistan, Mali, Rwanda and
dozens of other developing countries. When it does–in the
form of deceptively simple, inexpensive injection devices–it's
easy to overlook. But what can't be overlooked is the impact
of auto-disable injection technology, not only in getting vaccines
to remote and rugged areas, but also in making progress
against unsafe immunization practices that risk transmitting
blood-borne pathogens.
Teaming with the World Health Organization (WHO)
and other international health agencies, BD developed the
first auto-disable devices in the 1980s. Initially, high cost
threatened to curtail widespread use. BD responded by focusing
its manufacturing expertise on simple designs that drove
the cost of each device down significantly. Over the years, in
excess of 2.5 billion immunizations have been administered
using BD SoloShot devices alone.
In its ongoing effort to make injections safe, BD is leveraging
its global reach and resources. BD engineers and BD scientists
in India, China, Singapore, Brazil, Spain and the U.S. all
contributed to BD's portfolio of devices for safer injection,
including devices for clinical uses beyond immunization, for
emerging countries. BD's global presence gives the Company
on-the-ground support capabilities in Asia, Africa and Latin
America. The Company has also collaborated with a global
consortium of international agencies, government agencies,
nongovernmental organizations and industry leaders to
address safe injection practices and provide solutions.
Additionally, BD's worldwide manufacturing expertise continues
to enable the Company to produce devices at moderate
prices that support the sustainability of long-term immunization
programs.
In the future, newer devices using advanced medical technology
promise to make immunizations even more effective.
One such device, still under development, is the BD Intradermal
Delivery System. Its tiny microneedle penetrates the intradermal
layer immediately under the outermost epidermal skin layer.
BD scientists have found that certain drugs delivered at this
layer get into the blood stream faster and at lower doses than
standard injections. The microneedle itself is a major innovation,
as thin as a human hair and as short as 1 millimeter.
Those characteristics make it nearly pain-free, less threatening
and easy to use.
After smallpox emerged as a bioterror threat, BD drew
on its bank of knowledge and experience to release the
BD Bifurcated Needle for administering smallpox vaccine
in mass immunization campaigns and emergency response
situations. Given BD's commitment to preventing needlestick
injuries, the next step was to create a safety-engineered
version. Based on proven BD Eclipse technology, that device–
the BD Eclipse Bifurcated Needle with Safety Shield–features
single-handed activation, is easy to use and is economical.
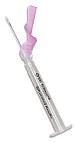 |
The BD Eclipse Bifurcated
Needle with Safety
Shield brings BD Eclipse
safety shielding to a needle
designed for delivery
of smallpox vaccine in
large-scale campaigns or
emergency responses. |
|
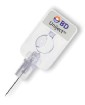 The BD Uniject prefill
injection device is a
single-use system, preventing
needle reuse
and eliminating the
need for filling syringes
from vials. Its innovative design allows for
fast and easy injections, while the compact
size allows easy transport, storage
and disposal. The BD Uniject prefill
injection device is a
single-use system, preventing
needle reuse
and eliminating the
need for filling syringes
from vials. Its innovative design allows for
fast and easy injections, while the compact
size allows easy transport, storage
and disposal. |
 The BD Intradermal Delivery
System features a tiny microneedle the
width of a human hair. Studies have shown that
delivery to the skin's intradermal layer can make
certain drugs more effective. The BD Intradermal Delivery
System features a tiny microneedle the
width of a human hair. Studies have shown that
delivery to the skin's intradermal layer can make
certain drugs more effective. |
 |
According to World
Health Organization
estimates, in the year
2000 alone, reused
medical devices led to
260,000 new cases of
HIV/AIDS, 2 million
hepatitis C infections and
21 million hepatitis B
infections. Mass immunization
programs represent
10 percent of all
injections administered
in the developing world.
BD is expanding its reuse
prevention efforts to
help address the other
90 percent of injections
administered to give
other medical care.
The BD SoloShot IX
and BD SoloShot LX
auto-disable syringes
expand BD's array of
auto-disable devices for
protecting children and
adults from unsafe injections.
The BD SoloShot IX
features a color-coded
plunger based on ISO
standards for quick,
sure identification of
the correct device. The
BD SoloShot LX is for the
tuberculosis vaccine and
other low-dose vaccines. |
|
|
|
|



