2012 Annual Report
Dear Fellow Shareholders:
It’s a pleasure to write my first letter to you as CEO. First, I want to thank the BD associates around the world for their efforts which were the foundation of a successful year. Their passion for our purpose of “Helping all people live healthy lives” was unabated. While the global business environment remained volatile and challenging in 2012, it did not prevent us from achieving our financial goals while also advancing our strategy, innovation, operating efficiency and talent management programs, which I believe provide a strong platform for future success. We also made some important strategic choices during fiscal year 2012, including the decision to sell the majority of our BD Biosciences – Discovery Labware unit.

Vincent A. Forlenza
Chairman, Chief Executive Officer and President
“Helping all people live healthy lives is about shared value: creating value for our shareholders by strengthening the health of communities and addressing the healthcare challenges of societies throughout the world.”
In fiscal year 2012, BD reported revenues of $7.708 billion and diluted earnings per share from continuing operations of $5.30, both of which met our expectations for the year.
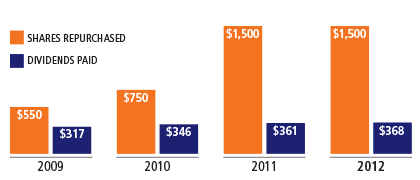
BD also returned $1.9 billion to our shareholders through a combination of share buybacks and dividends, as we increased our dividend for the 40th consecutive fiscal year. Our cash flow from operations totaled $1.7 billion, underlying our strong financial position.
Our performance this year was driven by strong performances in our BD Medical and BD Diagnostics segments. Our BD Biosciences business continues to be impacted in the U.S. by an uncertain research spending environment.
Our pipeline continues to mature as we launched 10 new products in 2012. International safety product sales grew 10.5% versus the prior year to $834 million. Acquisitions added about 100 basis points to our growth as we experienced strong customer acceptance of our BD PhaSeal, BD Accuri and BD Kiestra products.
Emerging market growth was also a positive contributor, reaching 23% of company sales, fueled by our second year of increased investment. Sales in China grew approximately 24.8% in 2012. We also see promising opportunities in India, Vietnam, Indonesia and Latin America.
Our Strategy
Dividends Paid vs. Shares Repurchased
($millions)
Our strategy is to make healthcare more effective, efficient and safer through innovation in areas that leverage the Company’s clinical knowledge and expertise. We focus primarily on improving parenteral drug delivery, including the management of diabetes; improving diagnostic testing, primarily for infectious disease and cancer; and improving technologies that enable researchers to understand the living cells and their functions. We see this as a global mission, collaborating with governments, non-governmental organizations and other stakeholders to create and deliver solutions to pressing healthcare challenges. We help to fund these efforts through a relentless focus on operating effectiveness.
We believe the principles of shared value are fundamental to our strategy. We provide essential value to society by helping address unmet health needs, and this in turn strengthens the Company’s businesses and creates new value for our shareholders. These principles are a foundation for the work we do around the globe, in both industrialized and emerging market settings, including resource-limited countries. We remain committed to sharing the benefits of this strategy through a return of capital to shareholders via dividends and share buybacks.
BD Innovations
BD Medical
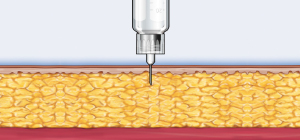
Many people with diabetes are hesitant or unwilling to give themselves insulin injections for reasons including needle anxiety. The BD Ultra-Fine Nano Pen Needle with PentaPoint Comfort, BD’s latest advancement in injection comfort, is a patented 5-bevel needle tip design that creates a flatter, thinner surface to help penetrate the skin with significantly greater ease. This will help enable patients to adhere more easily to therapy regimens recommended to improve their outcomes.
BD Diagnostics

Microbiology labs are facing unprecedented challenges while the need for faster
delivery of more accurate results is increasing to ensure optimal patient care. Through our acquisition of KIESTRA, the leader in Total Lab Automation, we have expanded our microbiology portfolio to include new automated instrumentation technologies that will enable us to offer innovative total lab automation solutions to laboratories worldwide.
BD Biosciences

Today, scientists familiar with flow cytometry choose dyes based on the number of surface receptors on the cells they are studying, as well as their brightness. We acquired Sirigen and its polymer dyes, which have the potential to add color choices and simplify flow for both expert and novice users. These new technologies enable a deeper level of biological study with more and brighter color choices for complex multicolor flow experiments.
Investing for the Future
“We have redirected more of our R&D spend…toward programs that will have a greater impact on improving patient outcomes and the efficiency of healthcare.”
In line with our belief that technology solutions can reduce healthcare costs and improve care, we invested $472 million in R&D and allocated $487 million of capital to new plants and equipment. We were pleased that multiple new product and technology programs progressed as planned. The BD MAX System, along with two assays for bacterial infections, was approved in the U.S. BD Biosciences launched the BD FACSJazz Cell Sorting System, which for the first time offers an extremely powerful tool for the identification and isolation of single or multiple cells, even from complex or extremely rare cell populations, right from the benchtop. The BD Nano Pen Needle, the world’s smallest pen needle, continued to find acceptance among people with diabetes looking for a better injection experience. We also made progress on our infusion collaboration with the Juvenile Diabetes Research Foundation (JDRF) and initiated a second collaboration to develop a continuous glucose sensor.
We complemented our internal innovation programs with strategic acquisitions, including KIESTRA Lab Automation. The combination of BD’s broad portfolio of microbiology platforms, reagents and supplies with KIESTRA’s automated instrumentation technologies will provide us with the technological foundation to offer innovative total lab automation solutions to hospitals and laboratories worldwide. We also acquired Sirigen Group Limited, a maker of flow cytometry dyes, which we believe will enable more complex experiments and faster results for our customers.
We believe that the healthcare environment has fundamentally changed in the developed world. Even when the global economy improves, we expect consumers and governments to continue to be more discerning buyers of healthcare. How are we meeting this challenge? First, we have redirected more of our R&D spend away from line extensions and toward programs that will have a greater impact on improving patient outcomes and the efficiency of healthcare. Second, we are also extending our reach into lower-priced emerging market segments with more price-competitive products, such as the new high-quality, low-cost BD Emerald Syringe line. Third, we are driving hard to lower our costs. I am proud to say that our ReLoCo cost reduction programs achieved their milestones during fiscal year 2012. We expect to realize incremental net cost savings of $40 to $50 million in fiscal year 2013.
Also, in 2012 we successfully implemented the first wave of EVEREST, our next-generation enterprise resource planning system, at a number of U.S. sites. Work has begun at the remaining sites, and we expect to complete the next set of implementations in the fall of 2013, with the program finishing in mid-2014. EVEREST, along with our network of shared service centers, will provide us with the systems to meet the cost and operating challenges of an increasingly global company.
Environmental Performance
“We received the WindMade™ label for our global operations, certifying that 35% of our total electricity use is from wind power.”
I am pleased to report continued progress this year in sustainable operations and product stewardship, which are the focus of BD’s environmental sustainability strategy. Our sites around the world have reduced energy, water and waste, contributing to progress against our 2015 Sustainability Targets.
We currently use 35% renewable energy in our operations, exceeding our 2015 renewable energy goal, and we have reduced energy consumption by 13% indexed to cost of goods sold against our goal of 30%. When combined with our renewable energy use, this has resulted in a 21.6% (absolute) reduction in greenhouse gas emissions. In addition, we reduced hazardous waste by 44% indexed to cost of goods sold, far exceeding our original goal of a 10% reduction.
BD is also proud to have become a WindMade™ Pioneer Company this year. WindMade is the first consumer labeling program to certify companies that source at least 25% of their power from wind energy. As a Pioneer Company, BD is among an elite group of organizations supporting the label, which will help us more effectively communicate our renewable energy use to customers and company stakeholders. Just recently, BD received the WindMade™ label for our global operations, certifying that 35% of our total electricity use is from wind power. We invite you to read more about our commitment to sustainability in BD’s Sustainability Report at www.bd.com/sustainability.
Global Health
Often, the way to make a significant difference in addressing global healthcare needs is through collaborations with organizations that have complementary skills, expertise and resources. This year, we collaborated with Heart to Heart International, a humanitarian medical aid nonprofit organization, for a second joint volunteer initiative to strengthen healthcare in Haiti. We also collaborated with Direct Relief International to vaccinate three million Haitian children for measles, rubella and polio in a campaign established by Haiti’s Ministry of Health. BD provided more than 1.7 million auto-disable immunization syringes and 150,000 sharps disposal containers.
In July, we announced a new collaboration, Labs for Life, with the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), through the Office of the U.S. Global AIDS Coordinator and the U.S. Centers for Disease Control and Prevention (CDC). The goal is to help strengthen healthcare and laboratory systems in countries highly impacted by the HIV/AIDS epidemic. Valued at $20 million, this collaboration builds on a prior five-year public-private partnership between BD and PEPFAR that focused on improving overall laboratory systems and services in targeted sub-Saharan African countries. This new collaboration will focus its investments in Uganda, Kenya, Ethiopia, Mozambique and India. Our ongoing work with PEPFAR has demonstrated how the private sector, in partnership with governments, can effectively apply its technologies and expertise to have a positive impact on healthcare in the regions most heavily burdened by infectious disease.
In the U.S., we collaborated with the Association for Professionals in Infection Control and Epidemiology (APIC), through the Heroes of Infection Prevention Award program and the Heroes Implementation Research Scholar Award. We believe that supporting research and learning will help ensure that the larger infection prevention community has the ability to replicate best practices in a broad range of healthcare settings.
Executive Appointments
We were very pleased to promote two new regional leaders to our executive team this year: Alex Conroy, President for Europe, EMA (Eastern Europe, Middle East and Africa) and the Americas, and James Lim, President for Greater Asia. Alex and James bring in-depth knowledge of these regions, and their insights will enable us to accelerate the globalization of BD. Additionally, Nabil Shabshab, who joined us in late 2011 as our Senior Vice President and Chief Marketing Officer, is making excellent progress driving customer insights much deeper into our planning, development programs and go-to-market efforts.
Key Board Developments
We are extremely pleased to welcome Rebecca Rimel, President and Chief Executive Officer of The Pew Charitable Trusts, to our Board of Directors. She brings to BD a unique blend of broad public policy expertise, philanthropic leadership and a strong healthcare background. She has already been an asset to the Board.
I would like to thank the Board, and in particular my predecessor, Ed Ludwig, who retired this year, for their guidance and support during this executive transition. While a change in leadership is significant in any company, Ed’s retirement marked only the sixth time in BD’s 115-year history that we have transitioned CEOs. I’m very grateful to Ed, as a mentor and a friend, for his help in preparing me and the Company for this transition.
In Closing
At BD, we understand that our greatest asset is the trust we earn, by fulfilling our commitments and being true to our purpose of Helping all people live healthy lives and doing so in accordance with our Core Values. We know we do not operate alone and we appreciate the support of our partners, customers and shareholders. Healthcare systems and patients all over the world are facing major difficulties. We believe we can help. I believe we have the right strategy and we are building the right capabilities to do our part to improve healthcare globally.
Thank you for the opportunity to lead this great company.
Vincent A. Forlenza
Chairman, Chief Executive Officer
and President
BD Around the World
North America
- New East Coast Distribution Center opens in Four Oaks, North Carolina.
- The BD Veritor System for rapid detection of Flu A+B delivers very good analytical sensitivity and specificity.
- In the U.S., BD offers BD Ultra-Fine Nano 4mm Pen Needles with PentaPoint Comfort.
- In the U.S., BD is donating insulin syringes and pen needles through Direct Relief to community health centers and free clinic partners.
South America
- The new BD Emerald Syringe product portfolio combines high-quality performance with a design that uses up to 30% less material than other syringes.
- In collaboration with the National Cancer Coalition, BD has committed to give 75,000 underserved Peruvian women access to BD SurePath liquid-based cytology tests over the next three years.
Europe
- The new safety-engineered BD Vacutainer Eclipse Signal Blood Collection Needle is now available.
- The BD MAX MRSA Assay has launched in Europe.
- New European Shared Service Center opened in Poland.
Africa
- BD, the Kenya Ministry of Medical Services and PEPFAR launched the Center for Excellence in Phlebotomy and Specimen Collection at the Kenya Medical Training College.
- Tanzania Initiative for Blood-Drawing Applications (TIBA), a multi-year BD collaboration with PEPFAR, aims to train healthcare workers to improve blood draw practices; expands needlestick injury prevention, surveillance and post-exposure management; and provides a framework to improve policies, guidelines and standard operating procedures.
Asia Pacific
- New R&D Center opened in India as part of BD’s efforts to accelerate innovation to develop new products.
- Public-private collaboration initiated in China to strengthen prevention and control of healthcare-associated infections.
