1987 | 1990s | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007

1991
Initial Public Offering (IPO) raises $54.4 million to support the Company’s ambitious plan to build an integrated biopharmaceutical company.
1993
Cephalon initiates clinical trials in the U.S. and files an Investigational New Drug (IND) application for modafinil for the treatment of excessive daytime sleepiness associated with narcolepsy.
The Company establishes a European presence in the United Kingdom (U.K.) and Ireland.

1994
The Company initiates a collaboration with TAP Pharmaceuticals to explore the use of the Company’s proprietary multilineage kinase inhibitors in the field of oncology.
1998
The Company’s first product, PROVIGIL® (modafinil) Tablets [C-IV], is approved in the U.S. and the U.K. It is the first new medication approved for narcolepsy in 40 years.
1999
Cephalon launches PROVIGIL, the first in a new class of wake-promoting agents, in the U.S.
2000
Cephalon acquires its first pain product, ACTIQ® (oral transmucosal fentanyl citrate) [C-II], and relaunches the product in the U.S.
ACTIQ is the first medication in the world approved for treating breakthrough pain in opioid-tolerant cancer patients.
Cephalon acquires U.S. product rights to GABITRIL® (tiagabine hydrochloride), a unique anti-seizure therapy and the first and only selective GABA reuptake inhibitor approved by the U.S. Food and Drug Administration (FDA).
2001
Cephalon receives authorization to market ACTIQ in 16 European countries.
The Company’s presence in Europe is dramatically expanded through the acquisition of the French pharmaceutical company Laboratoires L. Lafon and its largest selling product, the anti-spasmodic drug, SPASFON® (phloroglucinol).

2004
PROVIGIL is approved for additional indications by the U.S. FDA to improve wakefulness in adult patients with excessive sleepiness (ES) associated with shift work sleep disorder (SWSD) and treated obstructive sleep apnea (OSA).
The Company acquires CIMA LABS INC., a Minnesota-based drug delivery company with the first tablet formulation of the opioid fentanyl in development.
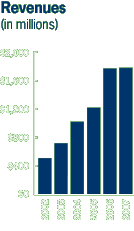
Cephalon revenues exceed $1 billion for the first time.
2005
Cephalon acquires TREANDA® (bendamustine HCI) for Injection, an oncology-targeted candidate in development for the treatment of hematologic malignancies, such as leukemia, lymphoma and myeloma.
The Company acquires TRISENOX® (arsenic trioxide) injection, a product approved in both the U.S. and Europe for treatment of relapsed or refractory acute promyelocytic leukemia (APL), a life-threatening hematologic cancer. The acquisition includes a dedicated hematology sales force in the U.S. and specialized oncology medical representatives in Europe.
Cephalon accelerates its entry into the European oncology market with the acquisition of Zeneus Holdings Ltd. The acquisition adds a direct presence in 19 countries in Europe and an oncology portfolio that includes MYOCET,® a liposomal formulation of doxorubicin for the treatment of metastatic breast cancer.
The Company enters a partnership with Alkermes to commercialize VIVITROL® (naltrexone for extended-release injectable suspension), a novel treatment for alcohol dependence, that expands the Company into a new therapeutic area.

2007
Cephalon files a marketing application with the European Agency for the Evaluation of Medicinal Products (EMEA) for its fentanyl buccal tablet formulation, under the trade name EFFENTORA,™ in Europe.
The U.S. FDA approves NUVIGIL™ (armodafinil) Tablets [C-IV] to improve wakefulness in patients with excessive sleepiness associated with treated OSA, narcolepsy and SWSD.
Additional clinical work initiated to explore the potential of NUVIGIL in a wide range of medical disorders.
Cephalon acquires the North American rights to AMRIX® (cyclobenzaprine hydrochloride extended-release capsules), a U.S. FDA-approved once-daily extended-release muscle relaxant.
Cephalon submits a supplemental New Drug Application (sNDA) to the U.S. FDA seeking approval to market FENTORA for the management of breakthrough pain in opioid-tolerant patients with chronic pain conditions other than cancer.
The U.S. FDA grants Orphan Drug Designation and priority review status to the New Drug Application (NDA) filing for TREANDA, an investigational treatment for chronic lymphocytic leukemia (CLL).
Cephalon files an NDA for TREANDA for treatment of patients with indolent (or slow growing) non-Hodgkin’s lymphoma (NHL) who have progressed during or following rituximab treatment.


