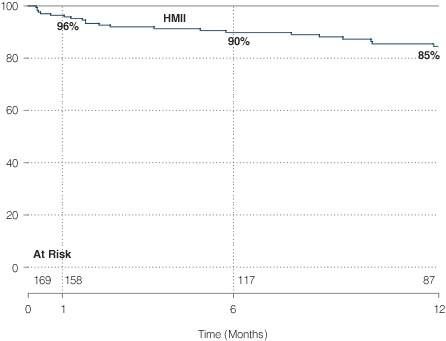
Kaplan-Meier Survival: HeartMate II
Bridge-to-Transplantation Post-Approval Study

2010 Annual Review
Kaplan-Meier Survival: HeartMate II
Bridge-to-Transplantation Post-Approval Study

After FDA approval of the HeartMate II for BTT in 2008, the first post-approval study utilizing the national Interagency Registry for Mechanically Assisted
Circulatory Support (INTERMACS) was initiated to
assess outcomes with the HeartMate II in a broader
patient care environment.
HeartMate II Left Ventricular Assist System Instructions for use #105747. Pleasanton, Calif: Thoratec Corp; Oct 2010.