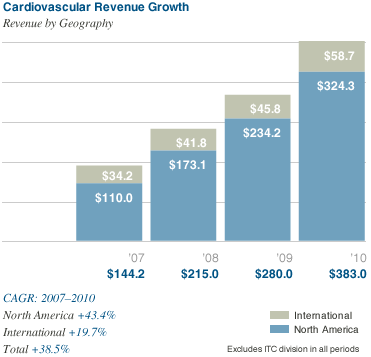
The Company generated a strong financial performance during the year, with
revenues from continuing operations of $383.0 million, an increase of 37 percent over 2009. We achieved a meaningful improvement to our bottom line as we realized
improved gross margin and operating leverage, while continuing to make significant investments in our programs designed to create long-term value for Thoratec. We also completed the disposition of International Technidyne Corporation, which provided us cash proceeds of $55 million and enabled the Company to increase its focus on the
MCS market opportunity.
We added 43 new HeartMate II centers during 2010, ending the year with 254, including 29 open-heart centers, which we believe will assume an increasingly important role in the utilization of MCS. In addition, a total of 90 centers in the U.S. had achieved Medicare certification for DT reimbursement from the Joint Commission at the end of the year, with a number of others in the final stages of the certification process.
Our new GoGear® HeartMate® external peripherals, introduced in late 2009,
experienced widespread adoption with more than 200 centers utilizing them at the end of 2010. These offerings, which include an improved battery, charger and power module, are providing patients increased freedom and mobility, and an enhanced quality of life. We continued the advancement of the HeartMate II platform with the full commercial launch of sealed inflow and outflow grafts in March 2011. By eliminating the need for pre-clotting, these grafts should reduce operating time, reduce costs, and lower intra-operative bleeding rates.
