We were also pleased with the outcomes from the BTT post-approval study, which
have been included in the HeartMate II product label and will be published in a major medical journal this spring. Compared to the BTT pivotal trial experience, the
post-market study data demonstrated continued efficacy and improving outcomes for HeartMate II BTT patients in the commercial setting, including significantly improved survival rates and very meaningful declines in the rate of adverse events—particularly stroke, device replacements and right heart failure. These findings are particularly impressive considering the fact that over 60% of the HeartMate II population was characterized as INTERMACS* profile I or II, indicating end-stage heart failure patients either in critical cardiogenic shock or in progressive decline on inotropes. It is also encouraging to note that these positive outcomes continue to be mirrored by the broad registry data being collected by INTERMACS.
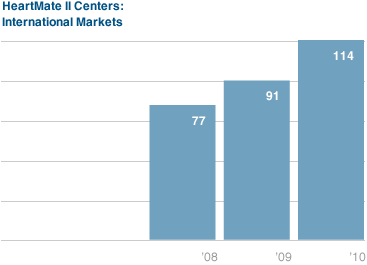
The HeartMate II also gained a stronger foothold in international markets, such as Europe and Asia Pacific, as we expanded our commercial presence to 35 countries as of year-end and grew our HeartMate II unit volume by 35 percent. We furthered our market leadership position in Europe, where we added a number of new HeartMate II centers and continued to drive the development of the DT market. And in Asia, our partner Nipro completed the confirmatory HeartMate II clinical trial in Japan and expects to make a regulatory submission in the first half of 2011. We also achieved regulatory approval in Australia and Taiwan for the HeartMate II for commercial use and experienced encouraging initial adoption of the device in Singapore, Malaysia, and Hong Kong.
* INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) is the national registry for patients receiving MCS therapy in the commercial setting.
