post-market studies that we believe will generate data demonstrating improved clinical and economic outcomes, while also providing support for expanding the patient
populations treated with VADs.
Product Development Pipeline
Our future product and technology development strategy is guided by three principles: continuing to evolve the HeartMate II platform to further our best-in-class technology position; pursuing the development of next-generation pumps; and introducing cross-platform breakthrough technologies that will substantially enhance the HeartMate II, as well as future pumps. We believe these efforts will create broader adoption of MCS, extend Thoratec’s market leadership position, and result in improved patient outcomes, enhanced quality of life, less invasive procedures and reduced cost of care.
During 2011, our plans for enhancements to the HeartMate II include the initial clinical utilization of a new controller that is smaller and lighter than currently available controllers and provides added convenience to the patients. We also expect to complete the development of and make a regulatory submission for new surgical implant tools used to attach the device to the left ventricle. These tools are designed to increase ease of use for surgeons and to reduce surgical and bypass time.
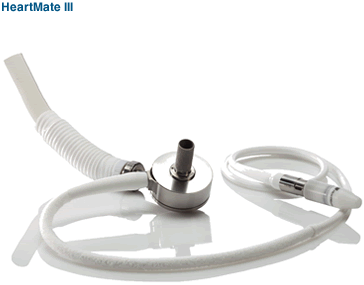
This year will be an important one in our development program for the HeartMate III. By combining the benefits of full magnetic levitation in a smaller pump that can be implanted less invasively, we believe the HeartMate III represents a breakthrough VAD technology that has the potential to provide significant clinical benefits, including reduced need for anti-coagulation, as well as reductions in the rates of thrombosis and bleeding. We have made great strides in the development of the pump’s key
