The goal of our drug delivery programs is to develop novel polymer products with the ability to deliver medicines in a targeted and controlled manner directly to the site in the body where they are required most. GLIADEL Wafer, intended for the treatment of brain cancer, is our first marketed product, but we are also developing new polymer products targeting a variety of other cancers Wafer, intended for the treatment of brain cancer, is our first marketed product, but we are also developing new polymer products targeting a variety of other cancers
|
|
 |
With our unique classes of biodegradable polymer products, Guilford is helping to advance the science of modern drug delivery by developing novel polymer drug delivery products for the treatment of cancer and other diseases. The specific purpose of Guilford’s polymer programs is to create products that administer treatment in a targeted and controlled manner. Because these products are site-specific, side effects are kept to a minimum, while efficacy is greatly enhanced.
GLIADEL Wafer Wafer
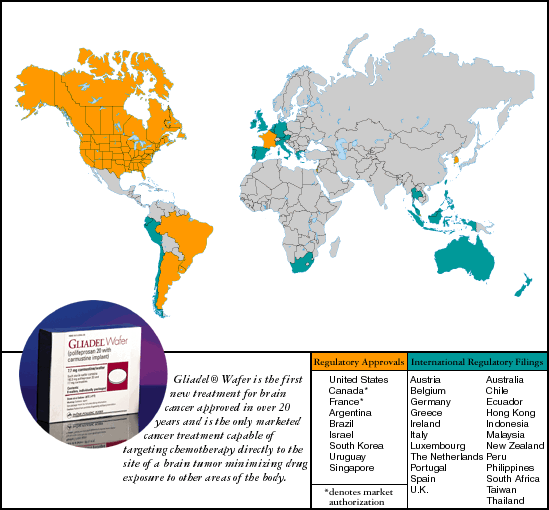
In 1996 the U.S. Food & Drug Administration granted permission to Guilford to commercially launch its first polymer-based product, GLIADEL Wafer for brain cancer. In 1997, in partnership with Rhône-Poulenc Rorer (RPR), Guilford made medical history by bringing to market the first and, to date, only cancer treatment capable of delivering chemotherapy directly to a tumor site. It represents the first significant advance in the treatment of brain cancer in two decades. Wafer for brain cancer. In 1997, in partnership with Rhône-Poulenc Rorer (RPR), Guilford made medical history by bringing to market the first and, to date, only cancer treatment capable of delivering chemotherapy directly to a tumor site. It represents the first significant advance in the treatment of brain cancer in two decades.
GLIADEL Wafer is currently approved for use in patients with recurrent glioblastoma multiforme, one of the most common and rapidly fatal forms of brain cancer. Once it is determined that surgery is an option for a patient, and after a tumor is surgically removed, up to eight dime-sized biodegradable GLIADEL Wafer is currently approved for use in patients with recurrent glioblastoma multiforme, one of the most common and rapidly fatal forms of brain cancer. Once it is determined that surgery is an option for a patient, and after a tumor is surgically removed, up to eight dime-sized biodegradable GLIADEL Wafers are inserted into the tumor bed. As it degrades, GLIADEL Wafers are inserted into the tumor bed. As it degrades, GLIADEL Wafer emits a precise dosage of chemotherapy directly to the tumor site. Studies reveal that GLIADEL Wafer emits a precise dosage of chemotherapy directly to the tumor site. Studies reveal that GLIADEL Wafer delivers 100 to 1,000 times more beneficial chemotherapy to a patient than standard methods of treatment. Moreover, GLIADEL Wafer delivers 100 to 1,000 times more beneficial chemotherapy to a patient than standard methods of treatment. Moreover, GLIADEL Wafer complements other standard therapies for brain cancer, such as surgery, radiation and traditional intravenous chemotherapy, without the serious side effects associated with these treatments. To date, over 3,000 patients worldwide have benefitted from treatment with GLIADEL Wafer complements other standard therapies for brain cancer, such as surgery, radiation and traditional intravenous chemotherapy, without the serious side effects associated with these treatments. To date, over 3,000 patients worldwide have benefitted from treatment with GLIADEL Wafer. Wafer.
Already approved in nine countries, Guilford and RPR are focusing their efforts on continuing to make GLIADEL Wafer available to more patients worldwide. In 1999, we expect additional regulatory approvals in Europe. Additionally, we are continuing a multinational Phase III clinical trial to assess the survival benefit of GLIADEL Wafer available to more patients worldwide. In 1999, we expect additional regulatory approvals in Europe. Additionally, we are continuing a multinational Phase III clinical trial to assess the survival benefit of GLIADEL Wafer when used at the time of first line therapy, with the goal of expanding the labeled indication for the product. Wafer when used at the time of first line therapy, with the goal of expanding the labeled indication for the product.
 |