66
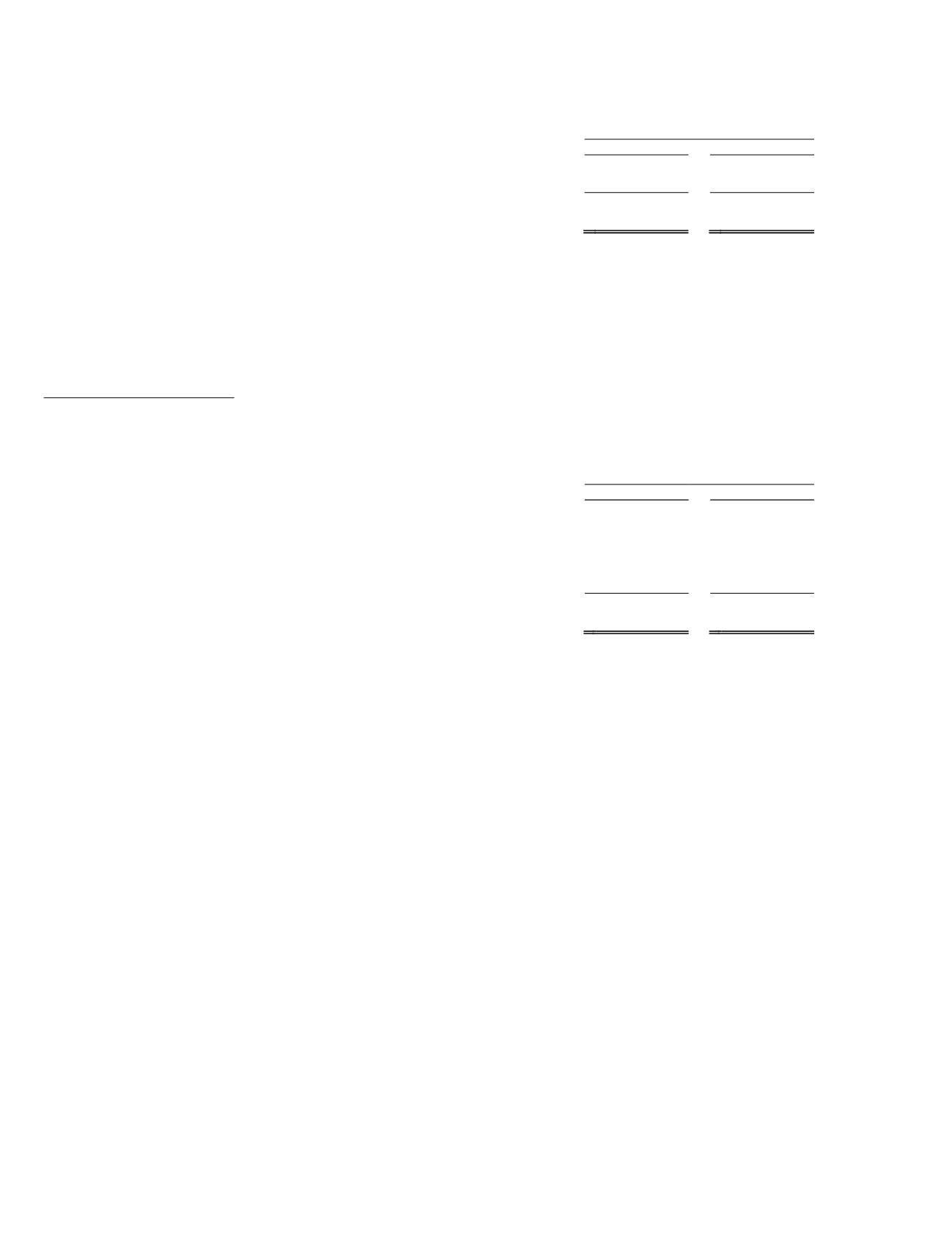
Our antisense drugdiscovery expenseswere as follows (in thousands):
YearEnded
December 31,
2013
2012
Antisense drug discovery expenses .............................................. $
42,402 $
34,035
Non-cash compensation expense related to equity awards ...........
2,878
2,108
Total antisense drugdiscovery ................................................. $
45,280 $
36,143
Antisense drugdiscovery costswere $42.4million for the year endedDecember 31, 2013 compared to$34.0million for
2012. Expenses increased in2013 compared to2012primarilydue to an increase in activities to support ourBiogen Idec and
AstraZeneca research collaborations, a $1.5million payment wemade toCHDI in the secondquarter of 2013, and additional supplies
used inour research activities. Under the terms of our agreementwithCHDI, we reimbursedCHDI for a portionof its support of our
Huntington’s disease programout of the $30million upfront payment we received fromour alliancewithRoche todevelop treatments
forHuntington’s disease. All amounts exclude non-cash compensation expense related to equity awards.
AntisenseDrugDevelopment
The following table sets forth expenses for ourmajor antisense drugdevelopment projects (in thousands):
YearEnded
December 31,
2013
2012
KYNAMRO ................................................................................. $
8,040 $
10,920
ISIS-TTR
Rx
...................................................................................
5,247
6,137
Other antisense development products .........................................
55,819
48,154
Development overhead costs ........................................................
8,689
5,350
Non-cash compensation expense related to equity awards ...........
3,202
2,482
Total antisense drugdevelopment ............................................ $
80,997 $
73,043
Antisense drug development expenditureswere $77.8million for the year endedDecember 31, 2013 compared to$70.6
million for 2012. The higher expenses in2013were primarilydue to an increase indevelopment costs associatedwith the progression
of numerous drugs inour pipeline into later stage clinical trials, including advancing ISIS-APOCIII
Rx
and ISIS-SMN
Rx
to the point
where each is poised tobeginPhase 3 studies. The increase associatedwith these activitieswas offset, inpart, by lower development
expenses related toKYNAMRO and ISIS-TTR
Rx
.We initiated a Phase 2/3 clinical studyof ISIS-TTR
Rx
inFebruary2013, forwhich
we incurred a significant portionof the start-up expenses in2012.We expect expenses for this study to increase as the study
progresses. As drugsmove forward tomore advanced stages of development, including into larger, longer clinical studies, such as our
plannedPhase 3 studies for ISIS-SMN
Rx
and ISIS-APOCIII
Rx
, the costs of development should increase. All amounts exclude non-
cash compensation expense related to equity awards.
Wemay conductmultiple clinical trials on a drug candidate, includingmultiple clinical trials for the various indicationswe
may be studying. Furthermore, aswe obtain results from trialswemay elect to discontinue clinical trials for certaindrug candidates in
certain indications inorder to focus our resources onmore promising drug candidates or indications. Our Phase 1 andPhase 2
programs are clinical researchprograms that fuel our Phase 3pipeline.Whenour products are inPhase 1or Phase 2 clinical trials,
they are in a dynamic state inwhichwe continually adjust the development strategy for eachproduct. Althoughwemay characterize a
product as “inPhase 1” or “inPhase 2,” it does notmean thatwe are conducting a single, well-defined studywithdedicated resources.
Instead, we allocate our internal resources on a sharedbasis across numerous products basedon eachproduct’s particular needs at that
time. Thismeanswe are constantly shifting resources amongproducts. Therefore, whatwe spendon eachproduct during a particular
period is usually a functionofwhat is required tokeep the products progressing in clinical development, notwhat productswe think
aremost important. For example, the number of people required to start a new study is large, the number of people required tokeep a
studygoing ismodest and the number of people required to finish a study is large. However, such fluctuations are not indicative of a
shift inour emphasis fromone product to another and cannot be used to accuratelypredict future costs for eachproduct. And, because
we always have numerous drugs inpreclinical and early stage clinical research, the fluctuations in expenses fromdrug todrug, in
large part, offset one another. Ifwe partner a drug, itmay affect the size of a trial, its timing, its total cost and the timingof the related
costs. As part of our collaborationwithGenzyme, we have transitioneddevelopment responsibility forKYNAMRO toGenzyme. We
andGenzyme share development costs equallyuntilKYNAMRO is profitable.


