

Diabetes and Obesity
Diabetes and obesity currently threaten the health, well-being and economic
welfare of virtually every developed country in the world. Worldwide,
morethan 150 million people currently live with diabetes. In the United
States, diabetes is the fifth leading cause of death by disease and contributes
to higher rates of morbidity including heart disease, blindness and kidney
failure. Obesity has risen at an epidemic rate during the past 20 years
with an estimated 300 million obese adults worldwide and another 18 million
children classified as obese. Obesity is a risk factor in many serious
conditions, and is the major risk factor contributing to type II diabetes,
with approximately 80% of cases directly related to obesity.
Over the last two years we have invested approximately $90MM in our UK-based
diabetes and obesity subsidiary, Prosidion. (OSI owns approximately 97%
of the shares of the subsidiary). Dr. Anker Lundemose, CEO of Prosidion,
has spent his career in both the pharmaceutical and biotech industries
working in this area and he has assembled a talented and experienced leadership
team based in our Oxford, UK facility. Prosidion is committed to the discovery
and early development of novel, next-generation small molecule compounds
to treat type II diabetes and obesity.


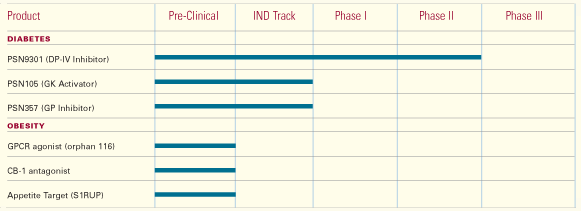
Prosidion has a strong and diverse early pipeline of projects and development
candidates in diabetes and obesity. Over the last year lead candidates
have been advanced from two discovery projects focused on the development
of small molecules designed to target glucokinase activation and glycogen
phosphorylase inhibition. Two of these candidates (PSN105 and PSN357)
are expected to enter clinical trials in the first half of 2005.
In June of 2004, we announced that Prosidion had successfully acquired
a Dipeptidyl Peptidase IV (DP-IV) platform, which included a Phase II
development candidate, P93/01, from the German company, Probiodrug. Dipeptidyl
Peptidase IV cleaves and inactivates Glucagon-like Peptide-1 (GLP-1),
an important mediator of blood glucose levels. Inhibition of DP-IV leads
to increased GLP-1 activity and the development of inhibitors of DP-IV
has become a very competitive arena for major pharmaceutical companies
active in diabetes. P93/01, re-named PSN9301, is an orally active, competitive
inhibitor of DP-IV and has been shown to lower glucose in type II diabetics
in clinical trials, and will enter further evaluation in Phase II trials
scheduled for early 2005. Prosidion also acquired a valuable intellectual
property estate in the DP-IV area that has been licensed on a non-exclusive
basis to several competitors in the field, including Merck and Novartis.
Prosidion will receive milestones and royalties on the successful development
and commercialization of DP-IV targeted drugs by these licensees.
While it is likely that Prosidion will seek a major pharmaceutical company as a commercialization partner for any diabetes drug that successfully emerges from its efforts in this area, we believe that the combination of expertise recruited into Prosidion and the subsidiary’s access to our full R&D support infrastructure, has resulted in a highly focused and dynamic research and development effort that will ultimately result in valuable new products in the treatment of diabetes and obesity and return considerable value to our shareholders.