



It is with great enthusiasm that we report to you our progress during
2004, without doubt the most remarkable year in the Company’s history
to date. At the heart of a year of transformation was the successful
demonstration that our flagship product Tarceva™ improved the survival
of patients in large, randomized Phase III clinical trials for advanced
lung and pancreatic cancers, widely recognized as amongst the toughest
cancers to treat. The results from the lung cancer trial led to the filing
of our first New Drug Application (NDA) at the end of July and the drug’s
subsequent approval by the U.S. Food and Drug Administration (FDA) on
November 18th – the fastest ever full approval by the FDA.
The Tarceva approval delivered on our long-standing commitment, to cancer
patients and shareholders alike, that we would bring this innovative new
medicine to market in a timely and comprehensive manner. The Tarceva success
has also positioned our oncology organization at the very forefront of
a paradigm-shifting change in the treatment of cancer patients –
away from the aggressive and toxic chemotherapy agents that have been
the mainstay of cancer treatment for decades and toward a new generation
of more targeted agents many of which, like Tarceva, can be taken as a
simple pill in the comfort of the patient’s home.
Even beyond the Tarceva approval, 2004 has been a productive year. Our
newly established commercial organization in oncology earned $34.5 million
in sales commission revenues for Novantrone®, some 15% more than our
assumptions entering the year. We were also able to both trigger our co-promote
rights with Genentech, Inc. (our U.S. partner for Tarceva) and participate
actively in the successful launch of Tarceva.
Beyond oncology, the cornerstone of our growing business, we have quietly
set about constructing a second disease area franchise in diabetes with
the investment of over $90MM in our UK-based subsidiary, Prosidion. The
acquisition of the dipeptidyl-peptidase inhibitor PSN9301, and its associated
intellectual property estate, from the German company Probiodrug AG over
the summer gave our diabetes franchise a high-quality Phase II clinical
development asset in what is clearly seen as one of the most topical areas
of research and development in diabetes today.
The decision in the spring to call the remaining $160 million of 4% convertible
senior subordinated notes issued in 2002 followed by the recently completed
$445MM secondary offering – the largest public equity offering in
the global biotech industry since 2000 – has improved our balance
sheet to such a degree that we are able to move forward with over $650MM
in cash and investments as we enter 2005.
This year has indeed been a remarkable one for OSI. We have established
ourselves amongst an elite group of biotech companies with proven capabilities
from discovery through to commercialization. The Company is anchored by
a strong financial foundation and is well positioned to capitalize on
Tarceva, a solid oncology organization and an emerging diabetes franchise.
Tarceva – Delivering on Expectations
Last November’s commercial launch of our flagship product, Tarceva,
marks the Company’s most significant milestone to date. Tarceva
is a potent, selective and orally active inhibitor of HER1/EGFR, a receptor
that is present, overexpressed or mutated in a wide variety of solid tumors
including those of the lung, brain, liver, ovary, head and neck and pancreas.
Tarceva was approved for use in the treatment of advanced non-small cell
lung cancer (NSCLC) patients after failure of at least one prior chemotherapy
regimen just three and a half months after OSI completed the filing of
the NDA. We believe that the speed of the FDA’s response and the
strength of the label we obtained are testaments to the compound itself,
to the quality of the NDA assembled by the OSI development and regulatory
teams (who took the lead role in this application on behalf of our partners,
Genentech and Roche) and to the professionals within the FDA, who have
committed themselves to respond proactively on review of drugs that address
major unmet clinical needs. Tarceva was launched less than three business
days after approval.
At the core of the Tarceva clinical success are two well-designed and
well-executed Phase III trials conducted by our colleagues at the National
Cancer Institute of Canada’s Clinical Trial Group in collaboration
with our own development group. Top-line results for the first of these
two trials were announced in April and showed that the study met its primary
endpoint of improving overall survival and its key secondary endpoints
of progression-free survival and objective tumor response in a 731-patient,
randomized, double-blind, placebo-controlled trial which compared single-agent
Tarceva to placebo in the treatment of patients with advanced NSCLC following
the failure of first- or second-line chemotherapy. This study formed the
basis of our recently approved NDA. In September, we announced the results
of a second Phase III study, this time in patients with locally advanced
or metastatic pancreatic cancer who had received no prior drug therapy.
The study, comparing Tarceva in combination with gemcitabine with chemotherapy
alone, surprised many observers by demonstrating a 23.5% improvement in
overall survival and meeting its primary endpoint. The positive outcome
of this trial was great news for pancreatic cancer patients and their
families. Tarceva is only the second agent ever to show the ability to
improve survival in this challenging disease setting. We hope to file
a supplemental NDA for Tarceva’s use for this indication in the
first half of 2005. Indeed, Tarceva is the only agent in the EGFR class
and the first non-traditional chemotherapy agent to demonstrate an improvement
in survival in either advanced NSCLC or pancreatic cancer.
Meanwhile, the recent failure of a competitor’s molecule to match
Tarceva’s demonstrated ability to improve survival in NSCLC has
further served to emphasize our long-held belief that Tarceva is a unique
agent well positioned to emerge as the best-in-class EGFR inhibitor. It
is now up to our tripartite alliance to continue to draw out the full
potential of the product. Key components of this effort in 2005 will be
the launching of the product in territories outside of the U.S. and the
expansion of an ongoing development program for Tarceva. We are pleased
that Roche, our partner for Tarceva outside of the United States, has
made significant progress on the European front. Roche completed the submission
of its filing to the European health authorities last August, and we anticipate
a launch for Tarceva in major European markets during the second half
of 2005.
Together with our partners, Genentech and Roche, we will pursue a comprehensive
development program designed to demonstrate the effectiveness of Tarceva
in earlier-stage lung cancer patients (in both front-line and adjuvant
disease settings), broaden utility of Tarceva to additional disease settings
(with the results in pancreatic cancer paving the way), and continue to
explore paradigm-shifting combinations of all targeted therapies, such
as Tarceva and Avastin®, for which we recently presented very intriguing
preliminary data in lung and renal cell cancers.
Establishing a Premier Oncology
Franchise
In March of 2003, we entered into an agreement with Serono S.A. to market
and promote Novantrone for oncology use in the United States. This was
a largely strategic move designed to allow the Company to transition itself
into a commercial organization in preparation for the launch of Tarceva.
Today we have an 80-person-plus core commercial organization including
sales, marketing, medical affairs, and commercial planning functions.
Notwithstanding the success of the commercial group in generating oncology
sales for Novantrone that have exceeded our expectations, our foresight
in building the group has allowed us to play an active role in launching
Tarceva and positions us as a potential partner-of-choice for prospective
alliances in the oncology arena as we move forward.


Our research and development efforts are increasingly focused on the development
of next-generation targeted therapies designed to supplement and complement
a portfolio built around Tarceva. OSI-930, a molecule designed as a co-inhibitor
of the c-kit and VEGFR tyrosine kinase receptors, is scheduled to begin
clinical trials in the first quarter of 2005 and OSI-817, a second development
candidate to arise from this program, is en route to begin clinical development
toward the end of the year. We were disappointed, but not surprised, to
report in June that Aptosyn®, an inducer of apoptosis (or programmed
cell death) that we acquired with the SAANDs technology platform from
Cell Pathways in 2003, did not meet its primary endpoint of improving
overall survival when used in combination with Taxotere® in patients
with advanced NSCLC. We continue to evaluate OSI-461, a more potent analog
of Aptosyn, and we continue to believe that apoptosis is an important
area of investigation in cancer research today. In fact, we have expanded
our efforts in this area beyond the SAANDs technology platform. Our research
programs have also established a network of alliances with outstanding
research institutions (such as Cold Spring Harbor Laboratory, where we
are using siRNA technology to identify and validate novel targets for
cancer drug discovery) and other biotechnology companies (such as Structural
Genomix, where we are employing crystallography techniques in structure-based
design approaches to drug discovery).
Although we believe that the future of our oncology franchise, and of
cancer treatment in general, will focus on these targeted therapies, chemotherapeutic
agents continue to be an important part of cancer treatment today. In
this respect, we are continuing the development of our differentiated
cytotoxic drug candidate, OSI-7904L. This candidate is a liposomal formulation
of a thymidylate synthase inhibitor and is designed to compete with the
most prominent agent in this class, 5-fluorouracil (5-FU). Our goal is
to mimic with a single, short-term infusion of our liposomal agent, the
sustained exposure achieved with long-term infusions of 5-FU. OSI-7904L
is being evaluated in a development program that includes Phase II trials
in gastric and biliary tract cancers and early combination studies with
cisplatin and oxaliplatin.
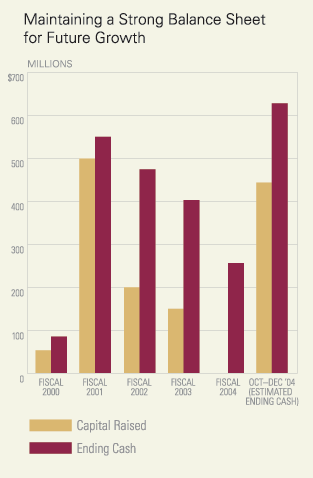

Building a Second Disease Area Franchise: Prosidion
We believe we have established the foundation from which to build a major biotechnology organization. However, we are convinced that achieving this goal will require us to build expertise and strength in more than one therapeutic area. As a result, Prosidion was established as a subsidiary of OSI in the United Kingdom in January of 2003 in order to provide a dynamic and independent vehicle to pursue research in the diabetes and obesity areas. While early partnering opportunities were explored, the dramatic progress of the group over the last 18 months coupled with the success of Tarceva and our financing efforts has allowed us to continue to nurture this valuable asset within the OSI framework.
Since early 2003 we have injected some $90MM of funding into the subsidiary, reached agreements with Tanabe Seiyaku Co., Ltd. (our former partner in funded diabetes research) and Lundbeck A/S that give us freedom-to-operate in our fields of interest in diabetes and obesity, and hired Dr. Anker Lundemose, an executive with extensive experience in the diabetes arena, who, as Chief Executive Officer of Prosidion, has assembled an outstanding management team at our Oxford, UK facility.
Our internal research efforts have led to the development of two lead candidate programs that target the activation of glucokinase and the inhibition of glycogen phosphorylase. We expect to begin clinical programs in both these areas during 2005. Finally, the acquisition of the dipeptidyl peptidase IV inhibitor, PSN9301, and a strong intellectual property estate from Probiodrug AG this summer has positioned Prosidion with an anchoring clinical program in one of the most attractive target areas in diabetes research and development today. Although we will likely need a major pharmaceutical industry partner to effectively commercialize these assets if they are successfully developed, we believe that, with over 90 million type II diabetics worldwide and a growing obesity problem in the western world, this investment represents a logical second disease area for our Company, which could realize significant value into the future.
Bringing it all Together: A Strong Financial Base and Striking the Right Balance
The success of Tarceva has given us the opportunity to establish a major biotechnology organization capable of delivering sustainable growth and value creation for our shareholders. In order to do this we will need to balance the goal of taking the business profitable around the anticipated commercial success of Tarceva with the continued investments in both our internal R&D and corporate development activities that will be necessary for us to fully capitalize on the business opportunity that is before us.
Maintaining a strong balance sheet and control of our core operating expenses are key financial requirements for our success. To this end, we took the difficult but necessary step of closing our oncology research efforts in the UK and consolidating them into our U.S.-based research operations in 2004. This has allowed us to continue our investments in Prosidion and expand our investments in translational research and commercial operations while maintaining a firm commitment to our stated goal of taking the company profitable within three years of Tarceva launch. With this level of investment in our ongoing business (we spent approximately $225MM on our core operations in 2004), we envision a robust OSI as we turn profitable – with a flagship product in Tarceva that continues to grow, a strong supplementary portfolio in our oncology business and the emerging commercial potential of a vibrant diabetes business. To ensure a strong capital base from which to build this organization we completed, in November, the largest secondary financing in the biotech sector since 2000 with a placement of 6,000,000 shares of our common stock (and a further 900,000 shares of common stock following the full exercise by the underwriters of their over-allotment option) at $64.50 per share. The Company ended the year with over $650 million in cash and investments.
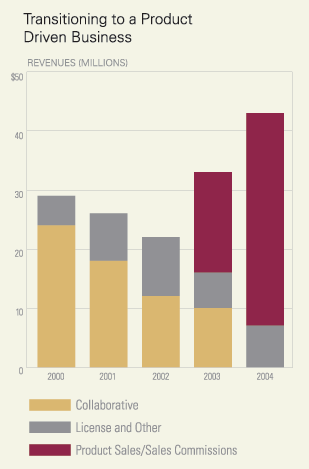
Our Commitment to an Exciting Future
There can be no doubt that this has been a banner year for OSI – clearly our best ever. We now have an opportunity to continue to build upon this base and take advantage of the success of Tarceva.
In closing, we also acknowledge that, in addition to the researchers and medical professionals who have contributed to the clinical success of Tarceva, none of our achievements would have been possible without the selfless contributions of the patients and their families who have participated, and continue to participate, in the many clinical trials we conduct each year aimed at improving the health of cancer and diabetes patients around the world.
Our mission remains the development of novel therapies that will shape the future of medicine and improve the lives of millions of patients worldwide and we are firmly committed to the belief that we can combine our mission with the goal of creating a commercially successful organization capable of creating sustainable growth and value for our patients, our stakeholders and our shareholders alike.
As we continue to develop our programs in oncology, diabetes and obesity, we will not lose sight of the fact that the partnership and commitment of our shareholders have been, and will continue to be, key to our success.
We would like to thank all of you who have supported OSI through your work, dedication and investment of time and money and we hope you will continue with us on our journey.
![]()
Colin Goddard, Ph.D.
Chief Executive Officer
Robert A. Ingram
Chairman of the Board
