


Targeting Cancer Pathways
Targeted therapies have the potential to change cancer from an acute and
often fatal disease to a chronic, manageable illness. These therapies
are designed to disrupt the “cellular pathways,” or the flow,
from the outside of the cell to the inside, of biological signals that
cancer cells use to grow, divide, repair themselves and communicate. Targeted
therapies are often more focused on the particular biology of the cancer
cell. This results in patients experiencing fewer of the toxic side effects
of traditional cytotoxic drugs, which usually harm normal tissues.
Tarceva – A Paradigm-Shifting Therapy
for
Cancer Patients Today
Tarceva is a small molecule inhibitor of the epidermal growth factor receptor,
or HER1/EGFR. Tarceva is designed as an oral once-a-day therapy to inhibit
the tyrosine kinase activity of the HER1 signaling pathway inside the
cell, which blocks tumor cell growth. We are developing and commercializing
Tarceva in a global alliance with Genentech and Roche.
In November of 2004, the U.S. Food and Drug Administration (FDA) approved
Tarceva for use as a monotherapy in the treatment of advanced non-small
cell lung cancer (NSCLC) patients who have failed at least one prior chemotherapy
regimen. OSI and its partner Genentech initiated the launch of Tarceva
less than three business days after its approval, making Tarceva available
to lung cancer patients immediately. By year end, Tarceva had captured
over 60% of all new prescriptions for EGFR targeted drugs in lung cancer.
The Tarceva approval was based on results from the BR.21 study that were
presented in full at the 2004 meeting of the American Society of Clinical
Oncology (ASCO) meeting in June. These results showed that the study met
its primary endpoint of improving overall survival and its key secondary
endpoints of progression-free survival and objective tumor response in
a 731-patient, randomized, double-blind, placebo-controlled trial which
compared single-agent Tarceva to placebo in the treatment of patients
with advanced NSCLC following the failure of first- or second-line chemotherapy.
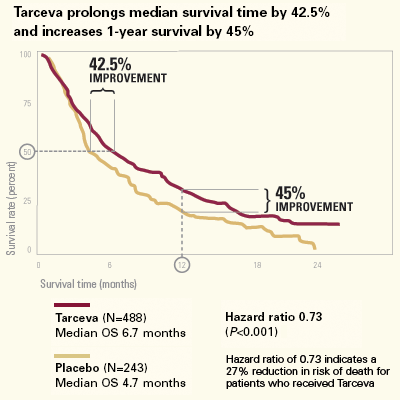
Tarceva improved overallsurvival in the study, with a hazard ratio of
0.73 (hazard ratio (HR) is a measure of the risk of death and a hazard
ratio of less than one indicates a survival benefit) and also demonstrated
a survival benefit in essentially all subsets of patients examined, including
males and females, patients with adenocarcinoma and squamous cell histology,
patients with good as well as impaired performance status, and both smokers
and non-smokers. Median and one-year survival of the overall population
in the BR.21 study was improved by 42.5 percent (6.7 versus 4.7 months)
and 45 percent (31.2 versus 21.5 percent) respectively, and patients were
treated with Tarceva for an average of just over four months in the study
(23% of patients were on therapy for more than 6 months). Certain subsets
of patients, including never smokers and patients who had tumors determined
to be EGFR-positive, were seen to have a large survival benefit in response
to treatment with Tarceva. The subgroup of patients who never smoked had
a substantial survival benefit with a hazard ratio of 0.42. The subgroup
of smokers also had a survival benefit (HR = 0.87) despite the fact that
this group was also seen to have a 24 percent higher rate of Tarceva clearance
(higher clearance rates lead to lower levels of exposure to a drug).

Although patients with tumors determined to be EGFR-negative did not appear
to have a survival benefit the statistical variation on this small subset
of patients was large and a survival benefit in this group cannot be ruled
out. However, we believe that, in practice, an appreciable majority of
patients with relapsed NSCLC presenting for therapy will have tumors of
unknown EGFR status. Indeed, this represented the largest EGFR status
subgroup in the BR.21 study and this group of “EGFR-Unknown”
patients had a robust survival benefit (HR = 0.76). No EGFR diagnostic
test is validated or approved for use in lung cancer and the FDA has not
required EGFR testing prior to the initiation of therapy with Tarceva.
In the pivotal study, the principal side effects associated with Tarceva
were rash (in 75% of patients, with approximately 9% of patients exhibiting
grade 3/4 rash) and a generally mild-moderate diarrhea (in 54% of patients,
with approximately 6% of patients exhibiting grade 3/4 diarrhea).
In September of 2004, OSI announced the positive results of a second Phase
III study which evaluated Tarceva in pancreatic cancer, comparing Tarceva
in combination with gemcitabine versus chemotherapy alone in patients
with locally advanced or metastatic pancreatic cancer who had not received
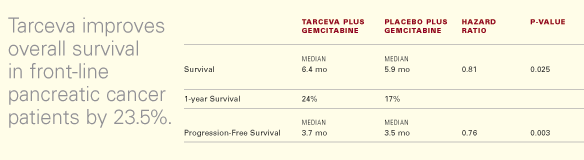
any prior drug therapy. The study met its primary endpoint of improving
survival, demonstrating a statistically significant (23.5%) improvement
in overall survival for patients receiving Tarceva plus gemcitabine when
compared to patients receiving gemcitabine plus placebo (HR = 0.81).
Each of the pivotal trials (BR.21 and the pancreatic Phase III program)
were sponsored by OSI and conducted by the National Cancer Institute of
Canada Clinical Trials Group at Queen’s University in collaboration
with our development group.
The ability of Tarceva to show a survival benefit in two cancers that
are widely recognized among the most difficult to treat suggests that
Tarceva has broad therapeutic potential in the treatment of cancer and
is clearly distinguished as a highly competitive agent within its class.
In addition, Tarceva has demonstrated indications of activity in head
and neck, ovarian, liver and brain cancers. Currently there are over 110
ongoing clinical studies, including a Phase IV development program to
seek expanded use of Tarceva to earlier-stage lung cancer patients (where
the compound has already shown indications of activity), additional disease
settings, and to develop all targeted therapy combinations as a truly
paradigm-shifting approach to cancer therapy. Studies of Tarceva used
in combination with Avastin in renal cell carcinoma have demonstrated
an encouraging initial response rate in the early stages of an ongoing
study, and a similar study is under way in NSCLC.
We and our partners are confident that Tarceva will emerge as the leader
in its class with the ability to provide a meaningful difference to the
lives of cancer patients around the world.

 Novantrone
is a synthetic antineoplastic anthracenedione used intravenously as an
anti-cancer agent. The FDA approved the product in 1987 for acute nonlymphocytic
leukemia (ANLL) and in 1996 for the relief of pain associated with hormone-refractory
prostate cancer (HRPC). It was also registered for multiple sclerosis
(MS) indications in October 2000 and is approved for use in the treatment
of Non-Hodgkin’s Lymphoma in markets outside the U.S. In March 2003,
OSI entered into an agreement with Serono to market and promote Novantrone
in the oncology marketplace in the United States.
Novantrone
is a synthetic antineoplastic anthracenedione used intravenously as an
anti-cancer agent. The FDA approved the product in 1987 for acute nonlymphocytic
leukemia (ANLL) and in 1996 for the relief of pain associated with hormone-refractory
prostate cancer (HRPC). It was also registered for multiple sclerosis
(MS) indications in October 2000 and is approved for use in the treatment
of Non-Hodgkin’s Lymphoma in markets outside the U.S. In March 2003,
OSI entered into an agreement with Serono to market and promote Novantrone
in the oncology marketplace in the United States.
While the transaction was executed largely as a strategy to allow us to
establish our commercial organization in preparation for the launch of
Tarceva, we are proud to participate in marketing a quality anti-cancer
drug like Novantrone to the oncology community. We now have an 80-person-plus
core commercial organization including sales, marketing, medical affairs
and commercial planning. We are also happy to report that we exceeded
our revenue goals for Novantrone by 15% generating $34.5 million in sales
commission revenues during fiscal year 2004. Our sales force will continue
to actively promote Novantrone through its patent expiration date of April
2006.

 Nearly
15% of all cancer patients who receive chemotherapy and more than 90%
of all patients receiving a combination of chemotherapy and radiation
therapy experience oral mucositis, a painful and often debilitating side
effect. Pain associated with oral mucositis can be so severe that patients
have difficulty eating and drinking. In 2003, through the acquisition
of Cell Pathways, we acquired the North American rights to Gelclair, a
bioadherent oral gel that provides rapid and durable relief of pain by
adhering to the mucosal surface of the mouth, forming a protective barrier
over the mouth and throat and thus shielding and soothing the exposed
and sensitized nerves. Although, as a device, Gelclair is not a major
product commercially, we will continue to offer this valuable treatment
option to cancer patients and their healthcare providers, who are dealing
with this painful side-effect of chemo- and radiotherapy.
Nearly
15% of all cancer patients who receive chemotherapy and more than 90%
of all patients receiving a combination of chemotherapy and radiation
therapy experience oral mucositis, a painful and often debilitating side
effect. Pain associated with oral mucositis can be so severe that patients
have difficulty eating and drinking. In 2003, through the acquisition
of Cell Pathways, we acquired the North American rights to Gelclair, a
bioadherent oral gel that provides rapid and durable relief of pain by
adhering to the mucosal surface of the mouth, forming a protective barrier
over the mouth and throat and thus shielding and soothing the exposed
and sensitized nerves. Although, as a device, Gelclair is not a major
product commercially, we will continue to offer this valuable treatment
option to cancer patients and their healthcare providers, who are dealing
with this painful side-effect of chemo- and radiotherapy.
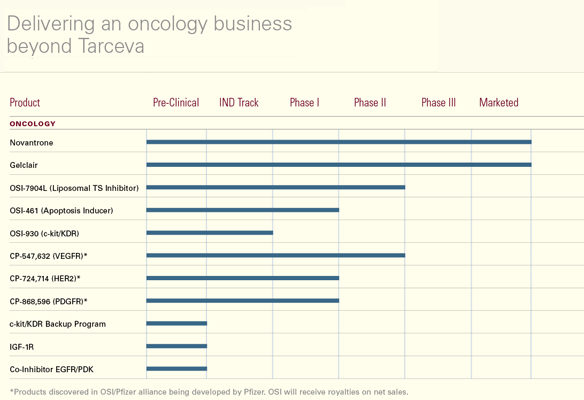
Beyond Tarceva – A
Franchise Delivered
The successful FDA approval and subsequent launch of Tarceva has established
a substantial corporate presence for OSI in the oncology arena. Our strategy
over the last several years has been focused on assembling all of the
pieces of a puzzle that adds up to a first-rate oncology organization
built around Tarceva.
We believe that we have achieved this goal and that only minimal infrastructural
investments will be required for the continued growth of our oncology
business. We believe it is essential that we continue to aggressively
manage our pipeline and to explore licensing and acquisition initiatives
designed to add oncology products and clinical candidates to our pipeline
in order to further strengthen our growing position in oncology.
We believe that in order to function as a successful oncology franchise
in the 21st century, we should focus on developing targeted therapies
as viable, next-generation options for patients who suffer from cancer.
Additionally, we consider the expansion into a second disease area to
be an important part of our strategy for long-term value creation. We
have therefore established Prosidion as a subsidiary of OSI with the mission
of establishing a portfolio of innovative diabetes and obesity products
that will make a meaningful impact on these growing healthcare threats.
